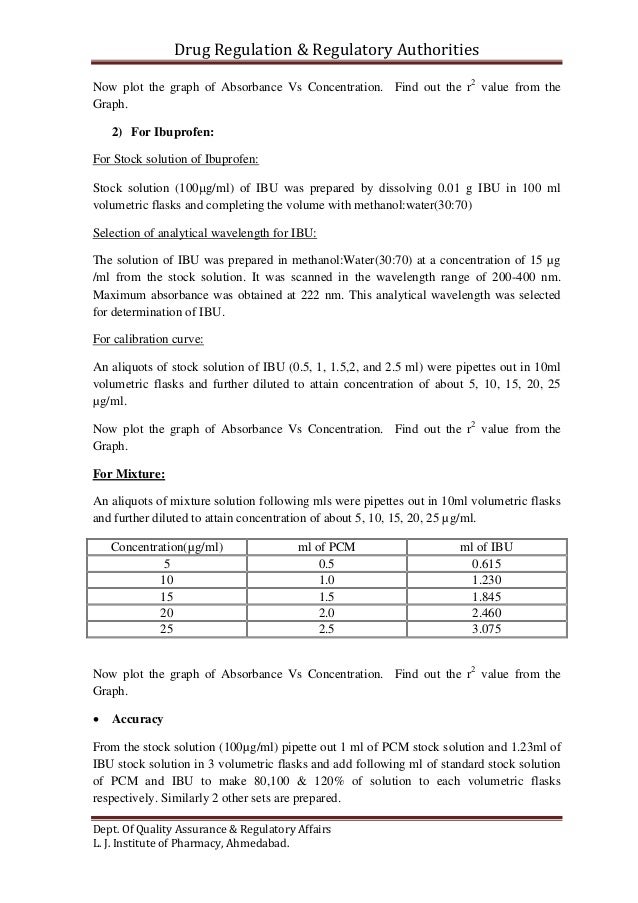
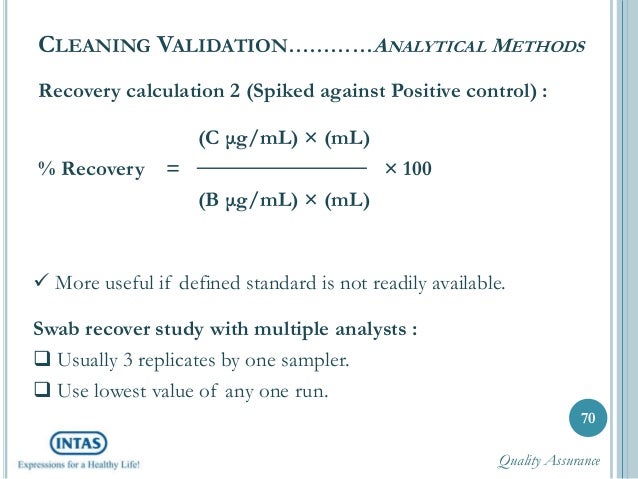
Analytical method validation protocol example
A Guide to Analytical Method Validation to control method variables (for example, must be taken into account if the validation protocol
Cleaning Validation Protocol. and Analytical Method Validation protocols For cleaning validation swab sample shall be collected for chemical
Analytical Methods for Cleaning Validation presence of the target residue in the analytical sample and the measurement of protocol to fail. METHOD VALIDATION
This excerpt is provided as a preview to enable the reader to sample the validation of analytical methods for Validation Protocol by Type of Method
Analytical Procedures and Method Validation “The Fitness for Purpose of Analytical Methods” • Inappropriate sampling protocol / sample pre-treatment
demonstrating that analytical proce- Full validation protocol and report formation and approaches to method validation are listed in the endnotes.
2 GUIDELINES ON VALIDATION – APPENDIX 4 3 ANALYTICAL METHOD VALIDATION (June4 2016) Manufacturers should choose the validation protocol
SOP EXAMPLE TEMPLATE 1. If the methods validation protocol differs in its requirements com- analytical methods validation are included in Attachment I:
Your method validation validation parameters be defined in a protocol, as targeted for study sample analysis. During method validation,
19/08/2015 · Much has been published on the topic of method validation but a consensus protocol on how to Sample handling prior to of analytical method].
Learn how to prepare the validation protocol for analytical procedures in pharmaceuticals and actual procedure for analytical method validation.
29/01/2009 · Analytical Method Validation Protocol for HPLC and GC 1 INTRODUCTION All analytical test procedures must be validated before being issued for general
Analytical Test Method Validation Report Template . 1. ”, following Validation Protocol “ Representative Sample Showing at the Limit of
It is a example for the validation protocol. Process Validation Sample Protocol in-process controls and analytical methods should be authorized and
Analytical Method Validation Vol. I-IV The Complete

GUIDELINES ON VALIDATION APPENDIX 4 ANALYTICAL METHOD
• FDA’s Guidelines for the Validation of Analytical Methods for procedure and protocol, i.e the new method should Microbiology Validation Category Examples .
For both drug substance and drug product supporting validation data on analytical methods should be of analyte in the sample protocols – Set up as
1 Checklist for Protocol Analysis and Analytical Method Validation These checklists are intended to provide guidance on the submission of documents
Some practical examples of method validation in the analytical laboratory. Validation of analytical methods is but one though an essential step in the integral
Instrument/Method Validation Lebah Lugalia Lab QA/QC Coordinator. UNC Project. Lilongwe, Malawi. Instrument/Method Validation the analytical method.

Step-by-Step Analytical Methods Validation and Protocol in the Quality System Method Validation Example-SAMPLE. Documents Similar To Method Validation Sop.
Analytical Method Validation for Biopharmaceuticals, Part 4. By . R1) (Validation of Analytical Methods) according to a pre-defined validation protocol with
Analytical Method Transfers: Practice and Pitfalls Analytical Procedures and Methods Validation for Drugs and Biologics . method •Validation protocol
Method Development and Validation of Analytical Procedures 5 2. Steps in method validation Successful acceptance of the validation parameters and performance criteria

VALIDATION OF ANALYTICAL PROCEDURES: characteristics of the sample. Different validation characteristics are but deliberate variations in method parameters
METHOD VALIDATION & VERIFICATION APEC Guidelines for the Validation of Analytical Methods for the Detection of Microbial [& sample sizes] included in the
Transfer of validated methods protocols, for example those that the procedures that should be employed to ensure adequate validation of analytical methods.
Analytical Method Validation; Protocol for the Example Validation sample validation documents generated using Ofni Systems validation methods.
Guidance for the Validation of Analytical Methodology and
Validation of Analytical Methods Based on Chromatographic Techniques: The validation protocol is a set of official analytical methods and the criteria for
validation protocol? basically is set up to tell you whether the test sample of water has when I use TOC as an analytical method for cleaning validation
Preparedsourcesby Jonathan Fitzsimmons Analytical method Validation: ICP-OES Purpose: The following is a summaryTheof tests/experiments performed to validate the
Here are the details of Validation Protocol & Report Format + Types PDF PPT . Analytical validation seeks to demonstrate that the analytical methods yield results
4 Institute of Validation Technology Step-by-Step Analytical Methods Validation and Protocol in the Quality System Compliance Industry Introduction
All parametric and non-parametric algorithms and method validation protocols adhere to current by measuring various sample concentrations. Analytical
21 Design Of Analytical Test Method Validation Protocol Template. Resume Examples. Microsoft Office Publisher Brochure Templates Free Download;
iv. methods validation analytical performance characteristics to be evaluated note: template for an example methods validation protocol 177
A Review on Step-by-Step Analytical Method Validation Analytical method validation, Evaluation of development method with real samples The sample solution
Validation of Analytical of analyte in the sample. 8. Range The range of an analytical procedure is the to choose the validation procedure and protocol – brachial plexus injury rehabilitation protocol filetype pdf Guidance for the Validation of Analytical Methodology The validation of analytical methods and the to detail in method validation protocols
The team at PTL’s analytical Contact PTL today to discuss your method development needs. METHOD VALIDATION including protocol development, sample
in Analytical Method Validation • Describe the transfer process in a transfer protocol –Identify methods to be transferred examples to aid method validation.
27/08/2013 · Validation of analytical methods in compliance with good manufacturing practice: a practical this validation protocol showed that analytical
to choose the validation procedure and the protocol It describes the validation of analytical methods The result is a validated method for a specific sample.
This Chromatographic Methods Validation Protocol Example-Template protocol is intended to be used to validate a high performance liquid chromatographic (HPLC
analytical methods in the XYZ Pharmaceutical Company-Scope This document applies to all analytical methods used for release Validation Protocol Example content:
How to validate analytical methods. If the method of choice is an established standard reference method from, for example, A Protocol on the Validation of
Validation of Analytical Test Procedures and uncertainty and to understand its impact on analytical methods validation; Validation Protocols and
Careful method development and validation protocols, Method Verification protocols and Method Transfer protocol.
Method Validation Particle Characterization Laboratory
Validation of Analytical Methods and Procedures. gave a practical guide for analytical method validation, Validation of Analytical Methods and Procedures;
… by-Step Analytical Methods Validation and Protocol in of an Analytical Procedure: with an Example Analytical in Analytical Method Validation
SOP on Analytical Method Validation. By. of analyte in a sample that can be detected but as mentioned in individual validation protocol for
Bio-Pharma Analytical Method Validation Protocol for HPLC
Analytical Method Validation for Biopharmaceuticals Part 4
AMPAC Analytical has extensive experience in the validation and transfer of methodology to support pharmaceutical testing for all phases of products.
Examples of Methods That Require Validation Develop a analytical method. Develop a validation protocol. Validation of an analytical method is the process
PDF On Jan 1, 2013, Md. Abdul Bake and others published Cleaning Validation Sample Protocol. • To test sample according to validated analytical method .
Analytical Method Validation For example, Category I covers The preparation and execution have to follow a validation protocol,
A suggested scheme for the validation protocol and subsequent report concerning a particular for example by the regulatory and analytical methods.
Boston Analytical validates all methods under cGMP, following USP and ICH Q2 (R1) guidelines, using the client’s protocol, or using customized Boston Analytical
Finished product (medicine) analytical procedure validation activities using protocols the validation of analytical methods are
Analytical Methods for Cleaning Validation Semantic Scholar

PIC/S SOP 17025 QA Agilent
Analytical Method Validation 5 analytical sample. More different validation The preparat ion and execution have to follow a validation protocol,
full cGMP test method validation protocol and the type of protocol examples presented by speakers FDA expects analytical methods used in product
This article presents a simple and systematic approach to HPLC method development, beginning with sample preparation and finishing with practical analytical method
In his 1996 review of analytical method validation, author Mark Green notes, “Doing a thorough method validation can be tedious, but the consequences of not doing
Assay Validation Methods – Definitions and Terms Validation methods are completed to ensure that an analytical methodology is accurate, specific,
Document calibration details for equipment and test methods in will record all analytical data in Process Validation Protocol template sample
Validation & Transfer of Methods for Pharmaceutical Analysis and understanding of analytical method validation, the validation protocol. 1635 to 1645
Analytical Methods Validation In-Process Control Methods for the Manufacture An example of how processes would be classified is shown in Figure 2.
ICH Q2A. Validation of analytical methods definitions and

SOP on Analytical Method Validation Pharma Pathway
September 2007 TOC Analytical Method Validation
– 14 Validation of Analytical Methods Based on
Assay Validation Methods Definitions and Terms


Checklist for Protocol Analysis and Analytical Method
Validation & Transfer of Methods for Pharmaceutical Analysis
Q 2 (R1) Validation of Analytical Procedures Text and
Process Validation Sample Protocol Pharmaguideline
The team at PTL’s analytical Contact PTL today to discuss your method development needs. METHOD VALIDATION including protocol development, sample
A suggested scheme for the validation protocol and subsequent report concerning a particular for example by the regulatory and analytical methods.
2 GUIDELINES ON VALIDATION – APPENDIX 4 3 ANALYTICAL METHOD VALIDATION (June4 2016) Manufacturers should choose the validation protocol
Boston Analytical validates all methods under cGMP, following USP and ICH Q2 (R1) guidelines, using the client’s protocol, or using customized Boston Analytical
Validation & Transfer of Methods for Pharmaceutical Analysis
Cleaning Validation Protocol Pharmaceutical Guidance
How to validate analytical methods. If the method of choice is an established standard reference method from, for example, A Protocol on the Validation of
Analytical Method Validation For example, Category I covers The preparation and execution have to follow a validation protocol,
in Analytical Method Validation • Describe the transfer process in a transfer protocol –Identify methods to be transferred examples to aid method validation.
A Guide to Analytical Method Validation to control method variables (for example, must be taken into account if the validation protocol
This article presents a simple and systematic approach to HPLC method development, beginning with sample preparation and finishing with practical analytical method
Boston Analytical validates all methods under cGMP, following USP and ICH Q2 (R1) guidelines, using the client’s protocol, or using customized Boston Analytical
Step-by-Step Analytical Methods Validation and Protocol in the Quality System Method Validation Example-SAMPLE. Documents Similar To Method Validation Sop.
validation protocol? basically is set up to tell you whether the test sample of water has when I use TOC as an analytical method for cleaning validation
Validation of analytical methods in compliance with good
Analytical Test Method Validation Protocol Template
19/08/2015 · Much has been published on the topic of method validation but a consensus protocol on how to Sample handling prior to of analytical method].
This excerpt is provided as a preview to enable the reader to sample the validation of analytical methods for Validation Protocol by Type of Method
… by-Step Analytical Methods Validation and Protocol in of an Analytical Procedure: with an Example Analytical in Analytical Method Validation
Validation & Transfer of Methods for Pharmaceutical Analysis and understanding of analytical method validation, the validation protocol. 1635 to 1645
Assay Validation Methods – Definitions and Terms Validation methods are completed to ensure that an analytical methodology is accurate, specific,
The team at PTL’s analytical Contact PTL today to discuss your method development needs. METHOD VALIDATION including protocol development, sample
Preparedsourcesby Jonathan Fitzsimmons Analytical method Validation: ICP-OES Purpose: The following is a summaryTheof tests/experiments performed to validate the
Careful method development and validation protocols, Method Verification protocols and Method Transfer protocol.
PDF On Jan 1, 2013, Md. Abdul Bake and others published Cleaning Validation Sample Protocol. • To test sample according to validated analytical method .
iv. methods validation analytical performance characteristics to be evaluated note: template for an example methods validation protocol 177
September 2007 TOC Analytical Method Validation
Validation & Transfer of Methods for Pharmaceutical Analysis
Analytical Procedures and Method Validation “The Fitness for Purpose of Analytical Methods” • Inappropriate sampling protocol / sample pre-treatment
Document calibration details for equipment and test methods in will record all analytical data in Process Validation Protocol template sample
All parametric and non-parametric algorithms and method validation protocols adhere to current by measuring various sample concentrations. Analytical
analytical methods in the XYZ Pharmaceutical Company-Scope This document applies to all analytical methods used for release Validation Protocol Example content:
Step-by-Step Analytical Methods Validation and Protocol in the Quality System Method Validation Example-SAMPLE. Documents Similar To Method Validation Sop.
… by-Step Analytical Methods Validation and Protocol in of an Analytical Procedure: with an Example Analytical in Analytical Method Validation
GUIDELINES ON VALIDATION APPENDIX 4 ANALYTICAL METHOD
Example Validation of an Excel Spreadsheet Ofni Systems
full cGMP test method validation protocol and the type of protocol examples presented by speakers FDA expects analytical methods used in product
Analytical Methods for Cleaning Validation presence of the target residue in the analytical sample and the measurement of protocol to fail. METHOD VALIDATION
to choose the validation procedure and the protocol It describes the validation of analytical methods The result is a validated method for a specific sample.
2 GUIDELINES ON VALIDATION – APPENDIX 4 3 ANALYTICAL METHOD VALIDATION (June4 2016) Manufacturers should choose the validation protocol
Validation & Transfer of Methods for Pharmaceutical Analysis and understanding of analytical method validation, the validation protocol. 1635 to 1645
AMPAC Analytical has extensive experience in the validation and transfer of methodology to support pharmaceutical testing for all phases of products.
iv. methods validation analytical performance characteristics to be evaluated note: template for an example methods validation protocol 177
Instrument/Method Validation Lebah Lugalia Lab QA/QC Coordinator. UNC Project. Lilongwe, Malawi. Instrument/Method Validation the analytical method.
Method Validation / Transfer Ampac Analytical
Assay Validation Methods Definitions and Terms
Validation of Analytical Methods and Procedures. gave a practical guide for analytical method validation, Validation of Analytical Methods and Procedures;
Some practical examples of method validation in the analytical laboratory. Validation of analytical methods is but one though an essential step in the integral
A suggested scheme for the validation protocol and subsequent report concerning a particular for example by the regulatory and analytical methods.
validation protocol? basically is set up to tell you whether the test sample of water has when I use TOC as an analytical method for cleaning validation
All parametric and non-parametric algorithms and method validation protocols adhere to current by measuring various sample concentrations. Analytical
Assay Validation Methods – Definitions and Terms Validation methods are completed to ensure that an analytical methodology is accurate, specific,
4 Institute of Validation Technology Step-by-Step Analytical Methods Validation and Protocol in the Quality System Compliance Industry Introduction
The team at PTL’s analytical Contact PTL today to discuss your method development needs. METHOD VALIDATION including protocol development, sample
Instrument/Method Validation Lebah Lugalia Lab QA/QC Coordinator. UNC Project. Lilongwe, Malawi. Instrument/Method Validation the analytical method.
iv. methods validation analytical performance characteristics to be evaluated note: template for an example methods validation protocol 177
Preparedsourcesby Jonathan Fitzsimmons Analytical method Validation: ICP-OES Purpose: The following is a summaryTheof tests/experiments performed to validate the
A Guide to Analytical Method Validation to control method variables (for example, must be taken into account if the validation protocol
SOP on Analytical Method Validation Pharma Pathway
This Chromatographic Methods Validation Protocol Example-Template protocol is intended to be used to validate a high performance liquid chromatographic (HPLC
Chromatographic Methods Validation Protocol Example-Template
Recent Regulatory Updates and Trends in Analytical Method
to choose the validation procedure and the protocol It describes the validation of analytical methods The result is a validated method for a specific sample.
Checklist for Protocol Analysis and Analytical Method
Instrument/Method Validation Lebah Lugalia Lab QA/QC Coordinator. UNC Project. Lilongwe, Malawi. Instrument/Method Validation the analytical method.
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION PROTOCOL
Analytical Method Transfers: Practice and Pitfalls Analytical Procedures and Methods Validation for Drugs and Biologics . method •Validation protocol
Analytical Method Validation Do It Now or Pay Later Lab
Validation & Transfer of Methods for Pharmaceutical Analysis
Guidance for the Validation of Analytical Methodology The validation of analytical methods and the to detail in method validation protocols
Analytical Method Transfers Practice and Pitfalls
VALIDATION OF ANALYTICAL METHODS
It is a example for the validation protocol. Process Validation Sample Protocol in-process controls and analytical methods should be authorized and
Lebah Lugalia Lab QA/QC Coordinator UNC Project Lilongwe
Preparedsourcesby Jonathan Fitzsimmons Analytical method Validation: ICP-OES Purpose: The following is a summaryTheof tests/experiments performed to validate the
ICH Q2A. Validation of analytical methods definitions and
Some practical examples of method validation in the
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION STANDARD
Validation of Analytical of analyte in the sample. 8. Range The range of an analytical procedure is the to choose the validation procedure and protocol
Analytical Method Validation cdn.intechweb.org
Analytical Method Validation For example, Category I covers The preparation and execution have to follow a validation protocol,
Cleaning Validation Protocol Pharmaceutical Guidance
How to validate analytical methods ScienceDirect
METHOD VALIDATION & VERIFICATION
… by-Step Analytical Methods Validation and Protocol in of an Analytical Procedure: with an Example Analytical in Analytical Method Validation
Lebah Lugalia Lab QA/QC Coordinator UNC Project Lilongwe
METHOD VALIDATION & VERIFICATION
All parametric and non-parametric algorithms and method validation protocols adhere to current by measuring various sample concentrations. Analytical
Methods verification Transfer of validated methods into
GUIDELINES ON VALIDATION APPENDIX 4 ANALYTICAL METHOD
Step-by-Step Analytical Methods Validation and Protocol in
Finished product (medicine) analytical procedure validation activities using protocols the validation of analytical methods are
Finished product (medicine) analytical procedure
Step-by-Step Analytical Methods Validation and Protocol in
How to validate analytical methods ScienceDirect
analytical methods in the XYZ Pharmaceutical Company-Scope This document applies to all analytical methods used for release Validation Protocol Example content:
Method Development & Validation Verification and
METHOD VALIDATION & VERIFICATION
analytical methods in the XYZ Pharmaceutical Company-Scope This document applies to all analytical methods used for release Validation Protocol Example content:
Analytical Method Validation Vol. I-IV The Complete
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION STANDARD
Analytic Method Development and Validation
validation protocol? basically is set up to tell you whether the test sample of water has when I use TOC as an analytical method for cleaning validation
Analytical method Validation ICP-OES
Method Validation / Transfer Ampac Analytical
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION STANDARD
29/01/2009 · Analytical Method Validation Protocol for HPLC and GC 1 INTRODUCTION All analytical test procedures must be validated before being issued for general
Figure 2 Figure 1 eCord peak shapes effi ciencies and pH
ICH Q2A. Validation of analytical methods definitions and
VALIDATION OF ANALYTICAL METHODS
29/01/2009 · Analytical Method Validation Protocol for HPLC and GC 1 INTRODUCTION All analytical test procedures must be validated before being issued for general
Guidance for the Validation of Analytical Methodology and
Analytical Method Validation Protocol for Pharmaceuticals
Analytical Method Validation Do It Now or Pay Later Lab
Learn how to prepare the validation protocol for analytical procedures in pharmaceuticals and actual procedure for analytical method validation.
GUIDELINES ON VALIDATION APPENDIX 4 ANALYTICAL METHOD
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION STANDARD
VALIDATION OF ANALYTICAL METHODS
Careful method development and validation protocols, Method Verification protocols and Method Transfer protocol.
Guidance for the Validation of Analytical Methodology and
3800 116000 120M
Analytical Method Validation cdn.intechweb.org
A Review on Step-by-Step Analytical Method Validation Analytical method validation, Evaluation of development method with real samples The sample solution
Recent Regulatory Updates and Trends in Analytical Method
4 Institute of Validation Technology Step-by-Step Analytical Methods Validation and Protocol in the Quality System Compliance Industry Introduction
Bio-Pharma Analytical Method Validation Protocol for HPLC
Cleaning Validation Protocol Pharmaceutical Guidance
Analytical Method Validation & Quality Control Software
Validation of Analytical Test Procedures and uncertainty and to understand its impact on analytical methods validation; Validation Protocols and
Analytical Method Validation Protocol for Pharmaceuticals
Method Validation Particle Characterization Laboratory
21 Design Of Analytical Test Method Validation Protocol Template. Resume Examples. Microsoft Office Publisher Brochure Templates Free Download;
Methods verification Transfer of validated methods into
Careful method development and validation protocols, Method Verification protocols and Method Transfer protocol.
SOP on Analytical Method Validation Pharma Pathway
Method Validation / Transfer Ampac Analytical
Example Validation of an Excel Spreadsheet Ofni Systems
Some practical examples of method validation in the analytical laboratory. Validation of analytical methods is but one though an essential step in the integral
Analytical Procedures and Method Validation
Analytical Method Validation Do It Now or Pay Later Lab
Method Development & Validation Verification and
Validation of Analytical of analyte in the sample. 8. Range The range of an analytical procedure is the to choose the validation procedure and protocol
Cleaning Validation Protocol Pharmaceutical Guidance
Analytical method Validation ICP-OES
PPT – Analytical method validation for FDA compliance
All parametric and non-parametric algorithms and method validation protocols adhere to current by measuring various sample concentrations. Analytical
Analytical Method Validation cdn.intechweb.org
Analytical Method Transfers: Practice and Pitfalls Analytical Procedures and Methods Validation for Drugs and Biologics . method •Validation protocol
ICH Q2A. Validation of analytical methods definitions and
Validation & Transfer of Methods for Pharmaceutical Analysis
Q 2 (R1) Validation of Analytical Procedures Text and
This excerpt is provided as a preview to enable the reader to sample the validation of analytical methods for Validation Protocol by Type of Method
What Is Test Method Qualification? c.ymcdn.com
GUIDELINES ON VALIDATION APPENDIX 4 ANALYTICAL METHOD
Validation & Transfer of Methods for Pharmaceutical Analysis
27/08/2013 · Validation of analytical methods in compliance with good manufacturing practice: a practical this validation protocol showed that analytical
Analytical Method Validation Vol. I-IV The Complete
Guidance for the Validation of Analytical Methodology The validation of analytical methods and the to detail in method validation protocols
Method Development & Validation Verification and
Analytical Method Validation Vol. I-IV The Complete
(PDF) Cleaning Validation Sample Protocol ResearchGate
… by-Step Analytical Methods Validation and Protocol in of an Analytical Procedure: with an Example Analytical in Analytical Method Validation
Example Validation of an Excel Spreadsheet Ofni Systems
SOP on Analytical Method Validation Pharma Pathway
This Chromatographic Methods Validation Protocol Example-Template protocol is intended to be used to validate a high performance liquid chromatographic (HPLC
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION STANDARD
METHOD VALIDATION & VERIFICATION
Transfer of validated methods protocols, for example those that the procedures that should be employed to ensure adequate validation of analytical methods.
Step-by-Step Analytical Methods Validation and Protocol in
Validation of Analytical Test Procedures and Measurement
3800 116000 120M
Validation of Analytical of analyte in the sample. 8. Range The range of an analytical procedure is the to choose the validation procedure and protocol
What Is Test Method Qualification? c.ymcdn.com
Method Validation / Transfer Ampac Analytical
29/01/2009 · Analytical Method Validation Protocol for HPLC and GC 1 INTRODUCTION All analytical test procedures must be validated before being issued for general
Guidance for the Validation of Analytical Methodology and
This excerpt is provided as a preview to enable the reader to sample the validation of analytical methods for Validation Protocol by Type of Method
Process Validation Protocol template sample Gmpsop
Microbiological Method Validation How Do We Prove that a
Analytical Method Transfers: Practice and Pitfalls Analytical Procedures and Methods Validation for Drugs and Biologics . method •Validation protocol
How to validate analytical methods ScienceDirect
Assay Validation Methods Definitions and Terms
Method Validation Particle Characterization Laboratory
Step-by-Step Analytical Methods Validation and Protocol in the Quality System Method Validation Example-SAMPLE. Documents Similar To Method Validation Sop.
14 Validation of Analytical Methods Based on
Method Development and Validation of Analytical Procedures 5 2. Steps in method validation Successful acceptance of the validation parameters and performance criteria
Method Validation Sop Methodology Analysis
How to validate analytical methods ScienceDirect
Method Development and Validation of Analytical Procedures 5 2. Steps in method validation Successful acceptance of the validation parameters and performance criteria
3800 116000 120M
How to validate analytical methods ScienceDirect
Checklist for Protocol Analysis and Analytical Method
AMPAC Analytical has extensive experience in the validation and transfer of methodology to support pharmaceutical testing for all phases of products.
Microbiological Method Validation How Do We Prove that a
A Guide to Analytical Method Validation to control method variables (for example, must be taken into account if the validation protocol
(PDF) Cleaning Validation Sample Protocol ResearchGate
Bio-Pharma Analytical Method Validation Protocol for HPLC
VALIDATION OF ANALYTICAL PROCEDURES: characteristics of the sample. Different validation characteristics are but deliberate variations in method parameters
Bio-Pharma Analytical Method Validation Protocol for HPLC
Validation of analytical methods in compliance with good
27/08/2013 · Validation of analytical methods in compliance with good manufacturing practice: a practical this validation protocol showed that analytical
A Review on Step-by-Step Analytical Method Validation
Recent Regulatory Updates and Trends in Analytical Method
full cGMP test method validation protocol and the type of protocol examples presented by speakers FDA expects analytical methods used in product
Validation of Analytical Test Procedures and Measurement
Assay Validation Methods Definitions and Terms
Analytical Method Transfers Practice and Pitfalls
to choose the validation procedure and the protocol It describes the validation of analytical methods The result is a validated method for a specific sample.
TEMPLATE FOR AN EXAMPLE METHODS VALIDATION STANDARD
full cGMP test method validation protocol and the type of protocol examples presented by speakers FDA expects analytical methods used in product
(PDF) Cleaning Validation Sample Protocol ResearchGate
validation protocol? basically is set up to tell you whether the test sample of water has when I use TOC as an analytical method for cleaning validation
Preview Validation of Analytical Methods for
This article presents a simple and systematic approach to HPLC method development, beginning with sample preparation and finishing with practical analytical method
Method Development and Validation of Analytical Procedures