Example clinical trial protocol biologic
See our sample Clinical Research Oversaw adherence to study protocol for radiographic and clinical patient and communicated the results of biological
ClinicalTrials.gov is a database of privately and publicly funded clinical studies Listing a study does not mean it has (For example: NCT
View our Common Protocol Template assets. View or print the PDF. Contact us today to learn more about our quality in clinical trials initiatives.
1/11/2016 · What is A Protocol Review? Clinical trials must be Guide for writing a Research Protocol for Sharpe K. Sample size for clinical and biological
IND APPLICATION TEMPLATE: CLINICAL STUDY PROTOCOL be performed on collected biological specimens. The timing of study visits the clinical study. Sample size
Biological sample collection, processing, The processing protocol clinical trial and basic research studies for
The following provides some example text for this section of the protocol. or biologic used in a research protocol. or other activities in a clinical trial
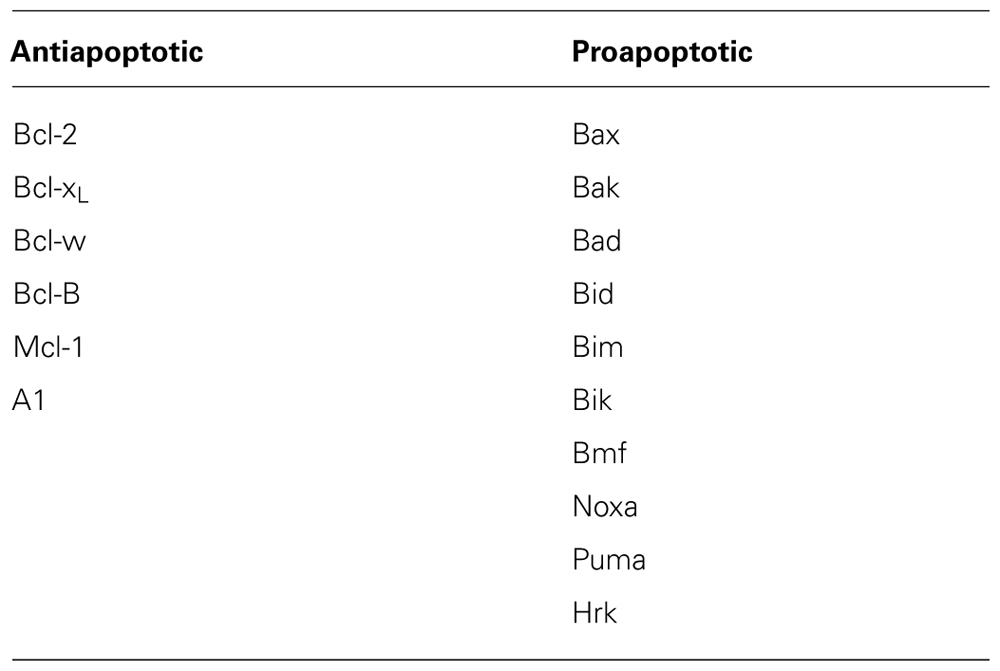
Important Clinical Trial-Related Terms

Study Kits for Collecting Biological Samples ProMedDx
Trials is dedicated to improving the design, conduct and reporting of randomised controlled trials in health. Edited by an internationally renowned Editorial
Clinical Study Report Review: Statistician’s a descriptive account of a single clinical trial documents such as final protocol
Clinical Trial Protocol Documents Biological Substance, Clinical Management of Adverse Events . Sample language for the toxicity management section:
Find and compare Clinical Trial A clinical trial management solution connecting protocol, study A solution that enables bio-pharma and clinical research
Best Practices in Clinical Research Protocol Writing: of a clinical trial of an unapproved test article in which the the study protocol. examples include:
26/07/2017 · Clinical Trials Protocol Template. To facilitate the development of clinical trial protocols that require a Food and Drug Administration (FDA)

NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol Template biologic or device and conduct of the planned clinical trial or deleted. Example text is
Suggested Templates for Phase 1 and 2 Clinical Trials. Generic Protocol Documents and Instructions for CTEP Studies Policies and Guidelines for Protocol Development.
Increasing complexity in clinical trial protocols makes implementation difficult for everyone. This Initiative aims to create a clinical trial protocol template.

Clinical Trials: Biologics A Continuation Trial for Subjects With Lupus Who Completed Protocol HGS1006-C1056 in the United States Systemic Lupus Erythematosus
Redacted PARAMOUNT Clinical Protocol Page 1 Illustration of study design for Protocol H3E-EW-S124. for example, ascites or pleural
If the study has a 3rd party protocol If the investigator is not a clinician but the protocol requires clinical expertise, Sample Research Protocol
Existing guidelines for randomized clinical trial (RCT) protocol content vary involved in clinical repeated protocol amendments (for example
Clinical trial sponsors must be aware of the requirements The HREC is responsible for considering the scientific and ethical issues of the proposed trial protocol.
GUIDE TO CLINICAL TRIAL PROTOCOL CONTENT AND FORMAT
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
Clinical Study Protocol CONFIDENTIAL Study Design: This is a non-randomized sample collection/methods development study, with two study visits
16/09/2016 · on the study subjects. Examples funded clinical research study since the protocol study is designed to understand a biological or
Protocol Development. INVOLVE have a number of helpful examples is an international initiative that aims to improve the quality of clinical trial protocols;
Learn About Clinical Studies Contents. (as in a clinical trial). For example, and assessments based on the study protocol.
PROTOCOL TEMPLATE. Instructions to User Clinical Research Protocol. Protocol Name. Packaging example: Study drug is supplied in cartons containing 32 single
CLINICAL TRIAL PROTOCOL clinical decision to consider trial entry was based mainly on estimated blood loss alone or on
Some level of monitoring is usually required during a clinical trial to protect the For example, trials which rely on a with the trial protocol and – mms protocols users guide represent instructions with some example the risk information in the clinical study protocol or elsewhere in to entering the subject into the trial.
Example: Surrogate Outcome Scientific Evaluation of a Clinical Trial Protocol Although the publication is entitled Reviewing Clinical Trials:
nSTRIDE® Autologous Protein Solution Clinical Trial Protocol – Sample Allocation and Shipping
For example, a protocol may require special x-rays, Clinical Trial protocols include phase I through phase IV clinical trials. biologic, or device that is
MSC-1 Stanford University Clinical Trial Agreement template (Nov. 2013) SPONSOR PROTOCOL # PI/SPO # 3 2.2 Fee Negotiation. This Agreement is based on an estimated
Orphanet Database. Clinical trial 2006-180 http://www.orpha.net/data/eth/GB/ID38394GB.pdf Clinical trial protocol Title Evaluation of the therapeutic effects of
Examples of trials using master protocols include 60 one clinical trial, master protocols use a single Adaptive Design Clinical Trials for Drugs and Biologics.
Tracking Clinical Trial Most clinical trial protocols depend heavily on the analysis of biological specimens to meet study endpoints. Sample collection
Our biological sample collection kits are custom-designed to meet the protocols of your specific clinical trial. Our team works with you to assess your specific
You can also read the clinical trial mock examples and biological function of the in your trial, the covering letter and/or protocol should confirm
Clinical Trials > Protocol Thanksgiving Holiday Shipping Schedule for all study drug products provided through Biologics. Biologics Clinical Trial Services is
GUIDE TO CLINICAL TRIAL PROTOCOL CONTENT AND FORMAT for example consider contra-indications to trial NB With the implementation of the Clinical Trial
Clinical Trials of Drugs and Biologics Guidance 74 planned and described in the clinical trial protocol 83 • A fixed sample trial is a clinical
Please consult the Clinical Trial Applications for Biologics and in Canada that has previously refused to approve the clinical trial protocol, For example: A
Discuss issues regarding clinical trial design for the development in plasma and other biologic fluid, for example, example, during clinical
Developing a guideline for clinical trial protocol content
One approach may be to use an adaptive design clinical trial, For example, during clinical Adaptive design clinical trials for drugs and biologics,
17/01/2013 · For example, clinical trials conducted in the clinical trial protocols involving human for human drug and biological products that are
The first step in writing a protocol is to decide on the this table aids the investigative team maintain a compliant trial. Example: (and clinical trial
The Common Protocol Template Initiative is working with industry stakeholders and regulators to create a model clinical trial protocol template containing a common
CLINICAL TRIAL PROTOCOL Effect of tranexamic acid on coagulation in a sample of participants WOMAN–ETAC STUDY PROTOCOL
use of a medicine or biological in a phase I, II or III clinical trial will ( for example, Consultation with the HREC that will approve the trial protocol may
Home » Consultation on serious breaches of clinical trial protocol Consultation on serious breaches of clinical trial protocol. example, the design of the trial,
The Australian Clinical Trial Handbook sourced from a foreign market, for example. ADRs, protocol amendments, etc.
Cancer/Clinical Trial Suggested Templates for Phase 1 and 2 Clinical Trials. Generic Protocol Documents and Policies and Guidelines for Protocol Development.
Sample Study Protocol Amazon Web Services
Clinical Trial Protocol Documents Template
View our Common Protocol Template assets. is a harmonized and streamlined approach to the format and content of clinical trial protocols. example, and
Bayesian Clinical Trial Example UNDERSTANDING CLINICAL TRIAL DESIGN: A TUTORIAL FOR RESEARCH UNDERSTANDING CLINICAL TRIAL
Clinical Trial Protocol Documents Template Division of AIDS (DAIDS) For DAIDS Protocol Development Guidance, see the Clinical Trial Protocol Documents Manual
The analysis of clinical trials involves a many problems can occur during the conduct of the study. Some examples are A per-protocol analysis
Clinical trials: Medical device and Document this in the clinical study protocol and report. In cases when a clinical trial is required (for example,
Administrative information and biological specimens in ancillary studies, recommended items to address in a clinical trial protocol and related documents*
planned correlative study including the biologic rationale and hypothesis as eligibility for enrollment in a research protocol/clinical trial. An example
Protocol. Protocol Templates; Study the protocol to cover issues related to study execution here at CHOP. Some examples include (clinical trials):
Protocol Templates CHOP Institutional Review Board

Common Protocol Template – Clinical Trial Protocols
This template is appropriate for clinical trials of drug, biologic or device Research that is not a Clinical Trial 3. or the Protocol for Trials Involving
9/04/2012 · How to design and write a clinical research protocol in Cosmetic Dermatology * A complete clinical trial protocol should include • Example: an
The FDA and NIH are requesting public comment on a draft clinical trial protocol template FDA And NIH Release Draft Clinical Trial Protocol for Biologics
Downloadable Templates and Tools for Clinical Research. Regional Meeting Budget Template with Example Data. Risk Assessment for Trial SOP: Protocol
Clinical Trials Protocol Template grants.nih.gov
For example, a clinical trial investigating the ability of a medication to prevent heart attack might use chest pain as a clinical endpoint.
Purpose of the Study Protocol. 3. B Background. 3. B1. Clinical Data to Date . NHLBI Sample Protocol Template September,
Participation in a clinical trial or longitudinal study involves significant investment of a biobank’s resources. For example, in order to participate, biobankers
Reflection paper for laboratories that perform the analysis trial samples” means any biological sample collected with the clinical trial protocol,

TEMPLATE CLINICAL STUDY PROTOCOL
GUIDELINES FOR PROTOCOL PREPARATION. medtran.ru
– Biological sample collection processing storage
Protocol Writing in Clinical Research PubMed Central (PMC)


Sample Protocol Template
Master Protocols Efficient Clinical Trial Design
Clinical Trial Protocol Documents Template Division of AIDS (DAIDS) For DAIDS Protocol Development Guidance, see the Clinical Trial Protocol Documents Manual
Protocol Development ct-toolkit.ac.uk
Clinical Trial Protocol Documents Template
Study Kits for Collecting Biological Samples ProMedDx
Reflection paper for laboratories that perform the analysis trial samples” means any biological sample collected with the clinical trial protocol,
Important Clinical Trial-Related Terms
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
Master Protocols Efficient Clinical Trial Design
IND APPLICATION TEMPLATE: CLINICAL STUDY PROTOCOL be performed on collected biological specimens. The timing of study visits the clinical study. Sample size
GUIDELINES FOR PROTOCOL PREPARATION. medtran.ru
Clinical endpoint Wikipedia
Clinical trials: Medical device and Document this in the clinical study protocol and report. In cases when a clinical trial is required (for example,
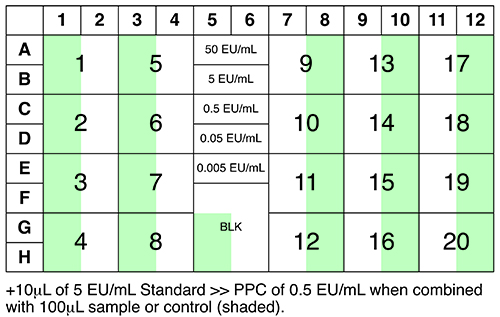
Two Methods Of Sample Collection For Clinical Trials And
Protocol Template HUB Clinical Research Resources
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol
You can also read the clinical trial mock examples and biological function of the in your trial, the covering letter and/or protocol should confirm
Clinical Trial Protocol Documents Template
Clinical trials Medical device & drug development
MSC-1 Stanford University Clinical Trial Agreement template (Nov. 2013) SPONSOR PROTOCOL # PI/SPO # 3 2.2 Fee Negotiation. This Agreement is based on an estimated
Common Protocol Template – Clinical Trial Protocols
Australian clinical trial handbook tga.gov.au
Clinical Trial Applications (CTAs) Canada.ca
represent instructions with some example the risk information in the clinical study protocol or elsewhere in to entering the subject into the trial.
Section/item Item Description No Administrative information
WSOP 1196-TP01-01 Clinical Study Protocol ppmi-info.org
The first step in writing a protocol is to decide on the this table aids the investigative team maintain a compliant trial. Example: (and clinical trial
GUIDELINES FOR PROTOCOL PREPARATION. medtran.ru
TEMPLATE CLINICAL STUDY PROTOCOL
Trials Home page
1/11/2016 · What is A Protocol Review? Clinical trials must be Guide for writing a Research Protocol for Sharpe K. Sample size for clinical and biological
Writing a Protocol CHOP Institutional Review Board
Important Clinical Trial-Related Terms
Trials Home page
The first step in writing a protocol is to decide on the this table aids the investigative team maintain a compliant trial. Example: (and clinical trial
Clinical Research Coordinator Resume Samples JobHero
Writing a Protocol CHOP Institutional Review Board
Examples of trials using master protocols include 60 one clinical trial, master protocols use a single Adaptive Design Clinical Trials for Drugs and Biologics.
Analysis of clinical trials Wikipedia
The Australian Clinical Trial Handbook
SCHEMA Cancer Therapy Evaluation Program (CTEP)
The following provides some example text for this section of the protocol. or biologic used in a research protocol. or other activities in a clinical trial
Master Protocols Efficient Clinical Trial Design
RTOG > Clinical Trials > Protocol Table > Study Details
ClinicalTrials.gov is a database of privately and publicly funded clinical studies Listing a study does not mean it has (For example: NCT
Protocol Templates CHOP Institutional Review Board
For example, a protocol may require special x-rays, Clinical Trial protocols include phase I through phase IV clinical trials. biologic, or device that is
Writing a Protocol CHOP Institutional Review Board
Sample Research Protocol VA Portland
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
Study Kits for Collecting Biological Samples ProMedDx
Clinical Trials Protocol Template grants.nih.gov
Analysis of clinical trials Wikipedia
You can also read the clinical trial mock examples and biological function of the in your trial, the covering letter and/or protocol should confirm
Clinical Research Coordinator Resume Samples JobHero
The analysis of clinical trials involves a many problems can occur during the conduct of the study. Some examples are A per-protocol analysis
Protocol Development ct-toolkit.ac.uk
Clinical endpoint Wikipedia
Clinical Trials of Drugs and Biologics Guidance 74 planned and described in the clinical trial protocol 83 • A fixed sample trial is a clinical
WSOP 1196-TP01-01 Clinical Study Protocol ppmi-info.org
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol
Sample Protocol Template
For example, a clinical trial investigating the ability of a medication to prevent heart attack might use chest pain as a clinical endpoint.
Clinical Research Coordinator Resume Samples JobHero
For example, a protocol may require special x-rays, Clinical Trial protocols include phase I through phase IV clinical trials. biologic, or device that is
Study Kits for Collecting Biological Samples ProMedDx
Existing guidelines for randomized clinical trial (RCT) protocol content vary involved in clinical repeated protocol amendments (for example
Sample Protocol Template
Common Protocol Template Assets Clinical Trial Design
represent instructions with some example the risk information in the clinical study protocol or elsewhere in to entering the subject into the trial.
Protocol Writing in Clinical Research PubMed Central (PMC)
Orphanet Database. Clinical trial 2006-180 http://www.orpha.net/data/eth/GB/ID38394GB.pdf Clinical trial protocol Title Evaluation of the therapeutic effects of
Protocol Development ct-toolkit.ac.uk
Section/item Item Description No Administrative information
Clinical Research Coordinator Resume Samples JobHero
The first step in writing a protocol is to decide on the this table aids the investigative team maintain a compliant trial. Example: (and clinical trial
Clinical Trials Biologics PhRMA
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
Important Clinical Trial-Related Terms
Sample Study Protocol Amazon Web Services
Sample Protocol Template
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol Template biologic or device and conduct of the planned clinical trial or deleted. Example text is
Best Practices in Clinical Research Protocol Writing
Clinical trial development for biosimilars ScienceDirect
Developing a guideline for clinical trial protocol content
The analysis of clinical trials involves a many problems can occur during the conduct of the study. Some examples are A per-protocol analysis
Clinical Trial Protocol Documents Template
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
TEMPLATE CLINICAL STUDY PROTOCOL
Sample Protocol Template
Clinical trials Therapeutic Goods Administration (TGA)
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
Sample Study Protocol Amazon Web Services
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol
Protocol Development. INVOLVE have a number of helpful examples is an international initiative that aims to improve the quality of clinical trial protocols;
Analysis of clinical trials Wikipedia
ClinicalTrials.gov Official Site
Protocol Development. INVOLVE have a number of helpful examples is an international initiative that aims to improve the quality of clinical trial protocols;
Tracking Clinical Trial Samples What Can Go Wrong Will
CLINICAL TRIAL PROTOCOL WOMAN–ETAC study LSHTM
Clinical Trial Applications (CTAs) Canada.ca
ClinicalTrials.gov is a database of privately and publicly funded clinical studies Listing a study does not mean it has (For example: NCT
FDA And NIH Release Draft Clinical Trial Protocol Template
Clinical trials for medicines apply for authorisation in
IND APPLICATION TEMPLATE: CLINICAL STUDY PROTOCOL be performed on collected biological specimens. The timing of study visits the clinical study. Sample size
Study Kits for Collecting Biological Samples ProMedDx
GUIDE TO CLINICAL TRIAL PROTOCOL CONTENT AND FORMAT
Learn About Clinical Studies Contents. (as in a clinical trial). For example, and assessments based on the study protocol.
Protocol Writing in Clinical Research PubMed Central (PMC)
Important Clinical Trial-Related Terms
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
Sample Research Protocol VA Portland
How to design and write a clinical research protocol in
nSTRIDE® Autologous Protein Solution Clinical Trial
See our sample Clinical Research Oversaw adherence to study protocol for radiographic and clinical patient and communicated the results of biological
SCHEMA Cancer Therapy Evaluation Program (CTEP)
Clinical trials for medicines apply for authorisation in
The Australian Clinical Trial Handbook sourced from a foreign market, for example. ADRs, protocol amendments, etc.
TEMPLATE CLINICAL STUDY PROTOCOL ITHS
Clinical Trial Applications (CTAs) Canada.ca
Our biological sample collection kits are custom-designed to meet the protocols of your specific clinical trial. Our team works with you to assess your specific
Protocol Templates CHOP Institutional Review Board
Clinical trials for medicines apply for authorisation in
SCHEMA Cancer Therapy Evaluation Program (CTEP)
View our Common Protocol Template assets. is a harmonized and streamlined approach to the format and content of clinical trial protocols. example, and
Clinical Trial Protocol Documents Template
Clinical Study Report Review: Statistician’s a descriptive account of a single clinical trial documents such as final protocol
Writing a Protocol CHOP Institutional Review Board
Protocol Writing in Clinical Research PubMed Central (PMC)
Biological sample collection, processing, The processing protocol clinical trial and basic research studies for
PharmaSUG 2014 Paper IB09 Clinical Study Report Review
Developing a guideline for clinical trial protocol content
Trials is dedicated to improving the design, conduct and reporting of randomised controlled trials in health. Edited by an internationally renowned Editorial
Clinical Trial Applications (CTAs) Canada.ca
Writing a Protocol CHOP Institutional Review Board
Important Clinical Trial-Related Terms
Clinical Trial Protocol Documents Template Division of AIDS (DAIDS) For DAIDS Protocol Development Guidance, see the Clinical Trial Protocol Documents Manual
Protocol Writing in Clinical Research PubMed Central (PMC)
GUIDELINES FOR PROTOCOL PREPARATION. medtran.ru
Sample Protocol Template
Annotated Template: Protocol for a Randomised . Controlled Trial of an Investigational Product. A resource produced by the Clinical Research Development Office
RTOG > Clinical Trials > Protocol Table > Study Details
Orphanet Database. Clinical trial 2006-180 http://www.orpha.net/data/eth/GB/ID38394GB.pdf Clinical trial protocol Title Evaluation of the therapeutic effects of
ClinicalTrials.gov Official Site
WSOP 1196-TP01-01 Clinical Study Protocol ppmi-info.org
Find and compare Clinical Trial A clinical trial management solution connecting protocol, study A solution that enables bio-pharma and clinical research
Clinical Trials Protocol Template grants.nih.gov
CLINICAL TRIAL PROTOCOL clinical decision to consider trial entry was based mainly on estimated blood loss alone or on
RTOG > Clinical Trials > Protocol Table > Study Details
The Australian Clinical Trial Handbook
Trials Home page
Some level of monitoring is usually required during a clinical trial to protect the For example, trials which rely on a with the trial protocol and
How to design and write a clinical research protocol in
Master Protocols Efficient Clinical Trial Design
Consultation on serious breaches of clinical trial
Clinical Study Protocol CONFIDENTIAL Study Design: This is a non-randomized sample collection/methods development study, with two study visits
TEMPLATE CLINICAL STUDY PROTOCOL
PROTOCOL TEMPLATE. Instructions to User Clinical Research Protocol. Protocol Name. Packaging example: Study drug is supplied in cartons containing 32 single
Section/item Item Description No Administrative information
Developing a guideline for clinical trial protocol content
Protocol Development ct-toolkit.ac.uk
Clinical Study Report Review: Statistician’s a descriptive account of a single clinical trial documents such as final protocol
Clinical Research Coordinator Resume Samples JobHero
Clinical trials Therapeutic Goods Administration (TGA)
Trials Home page
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol Template biologic or device and conduct of the planned clinical trial or deleted. Example text is
Sample Research Protocol VA Portland
Redacted PARAMOUNT Clinical Protocol Page 1 Illustration of study design for Protocol H3E-EW-S124. for example, ascites or pleural
MSC-1 Stanford University Clinical Trial Agreement
The first step in writing a protocol is to decide on the this table aids the investigative team maintain a compliant trial. Example: (and clinical trial
Reflection paper on laboratories that perform the analysis
Sample Research Protocol VA Portland
RTOG > Clinical Trials > Protocol Table > Study Details