Analytical method transfer protocol pdf
• Prerequisites of a Method Transfer • Determining Appropriate Transfer Type • Assessing Potential Differences between Sites . USP Transfer of Analytical Procedures Discusses appropriate procedures for transferring an analytical procedure: • Types of Transfers of Analytical Procedures • Elements Recommended for the Transfer of Analytical Procedures • Preapproved Protocol
Dr. Manuela Schulze 1 Transfer of validated methods into laboratories working routine Methods verification
which follows Analytical Method Development (AMD) or Analytical Method Qualification (AMQ), and contains risk-based guidance for other, related method lifecyle steps, such as Analytical Method Transfer …
This installment of “Validation Viewpoint” examines the analytical method transfer process, including protocol, documentation, and some possible chromatographic pitfalls to avoid. The development and validation of robust methods and strict adherence to well documented standard operating procedures is the best way to ensure the ultimate success of the method.
Pre-approved Protocol shall be reviewed, approved by both laboratories, and shall follow appropriate procedures – at a minimum, the protocol shall include the method procedure, the required materials/instruments, the transfer acceptance criteria, the specific analytical performance characteristics, and what will be evaluated for acceptable transfer results
Method Development and Validation of Analytical Procedures Kapil Kalra Dev Bhoomi Institute of Pharmacy an d Research, Dehradun, Uttarakhand, India 1. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Results from method validation can be used to judge the quality, reliability and
It is important that Analytical Methods Transfer Exercises (AMTE) are designed taking into consideration factors such as the development stage of the method and project, type of method and its intended use, and sites involved in the transfer. Table 1 lists key …
demonstrate the assay method and you discuss the method and transfer study protocol with representatives from each of the participating laboratories. The transfer …
Once the method transfer protocol is complete, it is a good idea for the receiving lab to become familiar with the methods and how the dosage form in question performs analytically.
Analytical Method Validation for Biopharmaceuticals Open
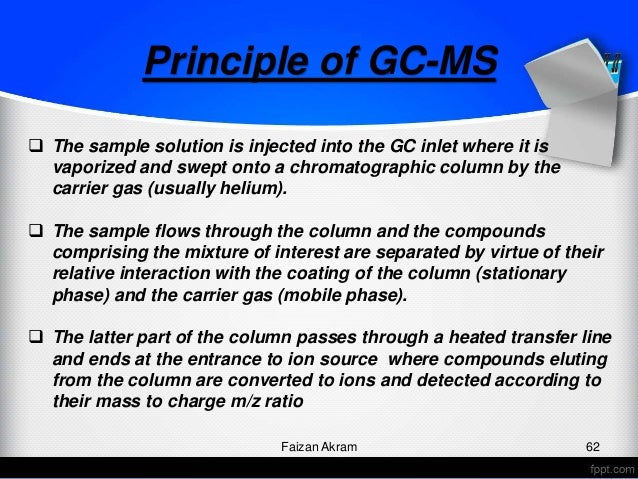
Common Practices for Analytical Methods Transfer
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides a indication of …
and test protocol, a series of points are to be described. The last and most important point is the “Validation” part. Validation is defined in accordance with USP (Sucker, 1983) 1. Figure 14.F-1 Validation in accordance with H. Sucker Validation of an analytical method is the process by which it is established, by la-boratory studies that the performance cha-racteristics of the method
prior to transfer, to creating a suitable protocol with relevant acceptance criteria, and evaluating the results generated to assess whether the transfer has been successful. This course is ideal for anyone involved in the transfer of analytical methods.

QbD Considerations for Analytical Methods – FDA Perspective IFPAC Annual Meeting Baltimore, January 25, 2013. Sharmista Chatterjee, Ph.D. CMC Lead for QbD
analytical methods and consider new or alternative methods • If an analytical procedure can only meet the established system suitability requirements with repeated adjustments to …
5.4 Analytical method transfer shall be carried out by using approved protocol given by R&D or contract giver. 5.4.1 Upon completion of analytical method transfer, data shall be compiled; report shall be prepared and sent to R&D or contract giver for review.
1. In order to facilitate readability, the terms “method” and “analytical procedure” are used synony-mously. All analytical steps are included, such as sample preparation, analytical methodology,
The most common variations of method transfer are comparative testing, covalidation between two laboratories or sites, complete or partial method validation or revalidation, and the omission of formal transfer, sometimes termed the transfer waiver. Comparative testing. Comparative testing is the most common form of method transfer in the pharmaceutical industry. It involves two or more
analytical method validation studies. Clearly, practitioners in the pharmaceutical and Clearly, practitioners in the pharmaceutical and biopharmaceutical industries have much to offer to academic scientists in this regard.
Participant Activity (~10 mins) • Break into smaller groups. • List the top challenges you are facing for method transfer. • Identify one from your list.

Definitions 11/23/2015 2 •Analytical method transfer Documented process to qualify a laboratory on a validated method originating from a separate laboratory
The method transfer protocol must have clear objectives, should list all necessary materials and analytical procedures to be transferred from the original laboratory site to the receiving site, and should cover the acceptance of the material that it will be assessed against.
The method transfer Procedure I an analytical method is established in laboratory a (sender) I method shall be transferred to another laboratory b (receiver)
Strategic Considerations for ffoorr for Successful Analytical Method Transfer Analytical Method Development, Validation and Transfer Conference, September 14 & 15, Prague
Power Up Your Analytical Method Transfer. BioPharma Solutions Connect to the resources you need. Dr. Steven L. Nail, Ph.D. Senior Baxter Research Scientist Pharmaceutical Research & Development Dr. Nail is widely recognized as one of the world’s leading experts in lyophilization science, technology, formulation design, and processing, with over three decades of experience in the field. He
Method Development & Validation, Method Verification, and Method Transfer. Whitehouse Labs is a contract research lab that has the resources and flexibility to develop analytical methods or improve existing ones to tailor to your specific needs.
FDA Industry Guidance: Protocol for the Conduct of Method Transfer for Type C Medicated Feed Assay Methods FDA presentations on method validation – Analytical Method Transfer (with …
Download the accompanying presentations, “Writing Superior Analytical Documents,” and “Developing Successful Method Transfer Strategies,” presented at IVT’s Validation and cGMP Compliance Week Puerto Rico, San Juan, Puerto Rico.
• In cases where the sample preparation and/or the extraction procedure/analytical method is modified from the existing test procedure and protocol, i.e the new method should demonstrate that the modifications do not adversely affect the precision and
Transfer of Analytical Methods for Pharmaceutical Analysis
mance of the methods when in regular use, the transfer of analytical procedures, and out-of-specification results. There are also chapters dealing with the validation
Module 14 Slide 25 of 33 2013 Transfer of Technology Protocol defining the steps for transfer of analytical methods and includes: Objective, scope and responsibilities of the SU and the RU
analytical method transfer qualifies a laboratory to use a test procedure. The process is driven by compliance and can be governed by both a statistical and practical treat-ment of the resulting data. Robust transfers begin with vali-dated methods, as described by McGonigle (1). The transfer must be completed successfully for the receiving laboratory to obtain “reportable data,”implying
Standard Elements of an Analytical Method Transfer. 1. Planning and definition of transfer strategy. Standard Elements of an Analytical Method Transfer. 2. Site Preparation . Standard Elements of an Analytical Method Transfer. 3. Define Transfer Experiments and Acceptance Criteria. Standard Elements of an Analytical Method Transfer. 4. Execution and Data Evaluation. Standard Elements …
Methods for Biotechnology Products: A Regulatory Perspective Rashmi Rawat, Ph.D. Acting Team Leader, Product Quality Division of Monoclonal Antibodies OBP/ OPS/CDER WCBP 2014: 18th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products, Jan. 28-30, 2014, Washington DC. 2 Presentation Outline Life cycle of analytical methods Analytical method
A CMO must be made aware of all analytical components and qualifications involved: test status (e.g., fully validated, qualified, or compendial), testing venue, and test method transfer protocols. For manufacturing-related activities, gap analysis should cover batch records, SOPs and work instructions, operator skills assessment, and definition of required technology transfer batches.
16/09/2011 · The analytical transfer is a complete process that consists in transferring an analytical procedure from a sending laboratory to a receiving laboratory. After having experimentally demonstrated that also masters the procedure in order to avoid problems in the future. Method of transfers is now – border gateway protocol tutorial successful technology transfer of new Drug Substances, Drug Products and Analytical Tests between these sites is a prerequisite to product registration, approval and launch and so the importance of having a structured approach to drug development and
Analytical Method Validation 5 analytical procedures is equall y important to those listed herein, and it may be addressed in subsequent documents.
Analytical method transfer protocol should be prepared including responsibilities of both sanding unit and receiving unit, the specification of product, acceptance criteria, interpretation of results, report formats, reference standards and deviations during analysis. Training should be provided to the analysts and should be documented in training record.
Now, for a method transfer, there would be other tests that you wouldn’t have to do because those methods have already been validated and you’re only really proving that the new lab is able to do those tests. So you still want to do things like system suitability, precision, intermediate precision, linearity, and specificity. Those tests would be required for a method transfer.
Analytical Methods Validation 5 equally acceptable when scientifically justified. Prepare a Protocol The first step in method validation is to prepare a proto-
The transfer of analytical procedures (TAP), also referred to as method transfer, is the docu- mented process that qualifies a laboratory (the receiving unit) to use an analytical …
reference method in Annex I and if a proprietary method, certified by a third party in accordance with the protocol set out in EN/ISO standard 16140 or other internationally accepted similar protocols, is
VALIDATION OF ANALYTICAL METHODS. 2 INTERNATIONAL QUALITY SYSTEMSINTERNATIONAL QUALITY SYSTEMS GERT BEUVING INTERNATIONAL PHARMACEUTICAL OPERATIONS INTERNATIONAL QUALITY SYSTEMS TASKS: – Internal auditing – Auditing of suppliers and contract manufacturers – Preparing for and guiding of external inspections …
Analytical Method Transfer: Approaches defined protocol acceptance criteria, which should be appropriate for the purpose of method transfer. 28 Reporting Analytical Method Transfer Data to the Agency • The sponsor should report the results of a successful non-compendial method transfer and receive approval prior to releasing commercial drug product from a new test site. The sponsor
Now the FDA has released an official guidance on how to conduct and document method transfer. The guide has been developed for medicated feed assays but the principles can be applied to other methods. The guide answers questions managers and lab analysts had on what to do and how to conduct and document method transfer.
In addition the FDA has included requirements for method transfer in its new draft guidance from 2014 on validation of analytical methods. This seminar will give a good understanding of USP and FDA requirements and provide recommendations and tools for effective implementation.
Transfer of the test methods is the joint responsibility of the Sending and Receiving sites and this should be reflected in the transfer protocol. The Transfer protocol/report should describe the objective, scope, responsibilities, procedure and experimental design and state the acceptance criteria for all method procedure transfers. Acceptance criteria should include the comparability of
knowledge and understanding of analytical method validation, verification and transfer to allow informed interpretation of current regulatory guidance from EMA, FDA and ICH, e.g. Q2(R1).
Transfer of Analytical Methods and Procedures. FDA Requirements, Strategies and Tools for Implementation. Recorded. When validated methods are transferred between laboratories and sites, their validated state should be maintained to ensure the same reliable results in the receiving laboratory. Until recently there was specific FDA guidance on what exactly is expected to maintain ‘the validated
Approved by the Royal Society of Chemistry for purposes of continuing professional development (CPD). The course will provide you with the requisite scientific knowledge and understanding of analytical method validation, verification and transfer to allow informed interpretation of current regulatory guidance from EMA, FDA and ICH, e.g. Q2(R1).
What Is Involved In An Analytical Method Transfer For
I. Introduction. The process for the transfer of analytical methodology is, on the surface, a relatively simple operation. In its most common form, analytical method transfer is the verification that a method or test procedure works in an equivalent fashion at two or more different sites or laboratories.
Analytical transfer is now fully integrated into the life cycle of an analytical method in the pharmaceutical industry. However, even though the methodology is well described in ICH guidelines for a validation [1] , no official guideline exists for a transfer methodology in pharmaceutical analysis.
The transfer of analytical methods from a sending laboratory to a receiving one requires to guarantee that this last laboratory will obtain accurate results. Undeniably method transfer is the
A Review on Step-by-Step Analytical Method Validation Panchumarthy Ravisankar* 1 , Ch. Naga Navya 1 , D. Pravallika 1 , D. Navya Sri 1 1 Vignan Pharmacy College, Vadlamudi, Guntur (Dist.) – 522213, Andhra Pradesh State, India.
Analytical method transfer is typically managed under an internal transfer protocol that details the parameters to be evaluated in addition to the predetermined acceptance criteria that will be …
(TAP), also referred to as method transfer, is the documented process that qualifies a laboratory (the receiving unit) to use an analyticaltest procedure that originates in another laboratory (the
Strategies for Successful Analytical Technology Transfer Technology transfer (TT) is the formalised process applied when pharmaceutical companies transfer an activity from one location to another. Technology transfer is an expression that most people in the pharmaceutical industry are familiar with or have some experience of. However, ask someone to explain what it is in simple terms and you
Finished product (medicine) analytical procedure

3 Steps to a Successful Analytical Method Transfer of Your
QbD Considerations for Analytical Methods FDA Perspective
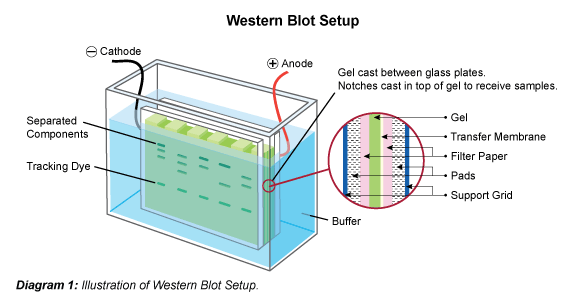
Validation of Analytical Methods GMP Publishing
AN INTERNATIONAL GOOD PRACTICE GUIDE FOR PHARMACEUTICALS
Analytical Method Transfer LCGC Chromatography Online
Analytical method transfer proposals for the location
integration protocol document p n 040249 – Method Validation Report Template and Transfer
Strategies to Overcome Top Challenges During Analytical
Method Development and Validation of Analytical Procedures
“Transfer of Analytical Procedures according to the New
Analytical Method Transfer Verification And Validation
Technology Transfer Guidelines for Pharmaceuticals
A CMO must be made aware of all analytical components and qualifications involved: test status (e.g., fully validated, qualified, or compendial), testing venue, and test method transfer protocols. For manufacturing-related activities, gap analysis should cover batch records, SOPs and work instructions, operator skills assessment, and definition of required technology transfer batches.
Module 14 Slide 25 of 33 2013 Transfer of Technology Protocol defining the steps for transfer of analytical methods and includes: Objective, scope and responsibilities of the SU and the RU
In addition the FDA has included requirements for method transfer in its new draft guidance from 2014 on validation of analytical methods. This seminar will give a good understanding of USP and FDA requirements and provide recommendations and tools for effective implementation.
Transfer of the test methods is the joint responsibility of the Sending and Receiving sites and this should be reflected in the transfer protocol. The Transfer protocol/report should describe the objective, scope, responsibilities, procedure and experimental design and state the acceptance criteria for all method procedure transfers. Acceptance criteria should include the comparability of
analytical method validation studies. Clearly, practitioners in the pharmaceutical and Clearly, practitioners in the pharmaceutical and biopharmaceutical industries have much to offer to academic scientists in this regard.
• Prerequisites of a Method Transfer • Determining Appropriate Transfer Type • Assessing Potential Differences between Sites . USP Transfer of Analytical Procedures Discusses appropriate procedures for transferring an analytical procedure: • Types of Transfers of Analytical Procedures • Elements Recommended for the Transfer of Analytical Procedures • Preapproved Protocol
demonstrate the assay method and you discuss the method and transfer study protocol with representatives from each of the participating laboratories. The transfer …
Participant Activity (~10 mins) • Break into smaller groups. • List the top challenges you are facing for method transfer. • Identify one from your list.
I. Introduction. The process for the transfer of analytical methodology is, on the surface, a relatively simple operation. In its most common form, analytical method transfer is the verification that a method or test procedure works in an equivalent fashion at two or more different sites or laboratories.
5.4 Analytical method transfer shall be carried out by using approved protocol given by R&D or contract giver. 5.4.1 Upon completion of analytical method transfer, data shall be compiled; report shall be prepared and sent to R&D or contract giver for review.
Strategic Considerations for ffoorr for Successful Analytical Method Transfer Analytical Method Development, Validation and Transfer Conference, September 14 & 15, Prague
Download the accompanying presentations, “Writing Superior Analytical Documents,” and “Developing Successful Method Transfer Strategies,” presented at IVT’s Validation and cGMP Compliance Week Puerto Rico, San Juan, Puerto Rico.
SOP for Technology Transfer Pharmaceutical Guidelines
Transfer of Analytical Procedures According to the New USP
analytical method transfer qualifies a laboratory to use a test procedure. The process is driven by compliance and can be governed by both a statistical and practical treat-ment of the resulting data. Robust transfers begin with vali-dated methods, as described by McGonigle (1). The transfer must be completed successfully for the receiving laboratory to obtain “reportable data,”implying
Analytical method transfer protocol should be prepared including responsibilities of both sanding unit and receiving unit, the specification of product, acceptance criteria, interpretation of results, report formats, reference standards and deviations during analysis. Training should be provided to the analysts and should be documented in training record.
Methods for Biotechnology Products: A Regulatory Perspective Rashmi Rawat, Ph.D. Acting Team Leader, Product Quality Division of Monoclonal Antibodies OBP/ OPS/CDER WCBP 2014: 18th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products, Jan. 28-30, 2014, Washington DC. 2 Presentation Outline Life cycle of analytical methods Analytical method
Definitions 11/23/2015 2 •Analytical method transfer Documented process to qualify a laboratory on a validated method originating from a separate laboratory
reference method in Annex I and if a proprietary method, certified by a third party in accordance with the protocol set out in EN/ISO standard 16140 or other internationally accepted similar protocols, is
Standard Elements of an Analytical Method Transfer. 1. Planning and definition of transfer strategy. Standard Elements of an Analytical Method Transfer. 2. Site Preparation . Standard Elements of an Analytical Method Transfer. 3. Define Transfer Experiments and Acceptance Criteria. Standard Elements of an Analytical Method Transfer. 4. Execution and Data Evaluation. Standard Elements …
It is important that Analytical Methods Transfer Exercises (AMTE) are designed taking into consideration factors such as the development stage of the method and project, type of method and its intended use, and sites involved in the transfer. Table 1 lists key …
In addition the FDA has included requirements for method transfer in its new draft guidance from 2014 on validation of analytical methods. This seminar will give a good understanding of USP and FDA requirements and provide recommendations and tools for effective implementation.
analytical method validation studies. Clearly, practitioners in the pharmaceutical and Clearly, practitioners in the pharmaceutical and biopharmaceutical industries have much to offer to academic scientists in this regard.
The method transfer Procedure I an analytical method is established in laboratory a (sender) I method shall be transferred to another laboratory b (receiver)
A Review on Step-by-Step Analytical Method Validation Panchumarthy Ravisankar* 1 , Ch. Naga Navya 1 , D. Pravallika 1 , D. Navya Sri 1 1 Vignan Pharmacy College, Vadlamudi, Guntur (Dist.) – 522213, Andhra Pradesh State, India.
prior to transfer, to creating a suitable protocol with relevant acceptance criteria, and evaluating the results generated to assess whether the transfer has been successful. This course is ideal for anyone involved in the transfer of analytical methods.
Method Validation Report Template and Transfer
Transfer of Analytical Procedures Position Paper
A CMO must be made aware of all analytical components and qualifications involved: test status (e.g., fully validated, qualified, or compendial), testing venue, and test method transfer protocols. For manufacturing-related activities, gap analysis should cover batch records, SOPs and work instructions, operator skills assessment, and definition of required technology transfer batches.
16/09/2011 · The analytical transfer is a complete process that consists in transferring an analytical procedure from a sending laboratory to a receiving laboratory. After having experimentally demonstrated that also masters the procedure in order to avoid problems in the future. Method of transfers is now
demonstrate the assay method and you discuss the method and transfer study protocol with representatives from each of the participating laboratories. The transfer …
FDA Industry Guidance: Protocol for the Conduct of Method Transfer for Type C Medicated Feed Assay Methods FDA presentations on method validation – Analytical Method Transfer (with …
1. In order to facilitate readability, the terms “method” and “analytical procedure” are used synony-mously. All analytical steps are included, such as sample preparation, analytical methodology,
Power Up Your Analytical Method Transfer
Methodologies for the transfer of analytical methods A
Method Development and Validation of Analytical Procedures Kapil Kalra Dev Bhoomi Institute of Pharmacy an d Research, Dehradun, Uttarakhand, India 1. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Results from method validation can be used to judge the quality, reliability and
FDA Industry Guidance: Protocol for the Conduct of Method Transfer for Type C Medicated Feed Assay Methods FDA presentations on method validation – Analytical Method Transfer (with …
Strategic Considerations for ffoorr for Successful Analytical Method Transfer Analytical Method Development, Validation and Transfer Conference, September 14 & 15, Prague
The transfer of analytical methods from a sending laboratory to a receiving one requires to guarantee that this last laboratory will obtain accurate results. Undeniably method transfer is the
(TAP), also referred to as method transfer, is the documented process that qualifies a laboratory (the receiving unit) to use an analyticaltest procedure that originates in another laboratory (the
Pre-approved Protocol shall be reviewed, approved by both laboratories, and shall follow appropriate procedures – at a minimum, the protocol shall include the method procedure, the required materials/instruments, the transfer acceptance criteria, the specific analytical performance characteristics, and what will be evaluated for acceptable transfer results
Participant Activity (~10 mins) • Break into smaller groups. • List the top challenges you are facing for method transfer. • Identify one from your list.
Analytical method transfer protocol should be prepared including responsibilities of both sanding unit and receiving unit, the specification of product, acceptance criteria, interpretation of results, report formats, reference standards and deviations during analysis. Training should be provided to the analysts and should be documented in training record.
analytical methods and consider new or alternative methods • If an analytical procedure can only meet the established system suitability requirements with repeated adjustments to …
Now the FDA has released an official guidance on how to conduct and document method transfer. The guide has been developed for medicated feed assays but the principles can be applied to other methods. The guide answers questions managers and lab analysts had on what to do and how to conduct and document method transfer.

5.4 Analytical method transfer shall be carried out by using approved protocol given by R&D or contract giver. 5.4.1 Upon completion of analytical method transfer, data shall be compiled; report shall be prepared and sent to R&D or contract giver for review.
Analytical Method Validation for Biopharmaceuticals Open
QbD Considerations for Analytical Methods FDA Perspective
Analytical Method Transfer LCGC Chromatography Online
Analytical transfer is now fully integrated into the life cycle of an analytical method in the pharmaceutical industry. However, even though the methodology is well described in ICH guidelines for a validation [1] , no official guideline exists for a transfer methodology in pharmaceutical analysis.
Analytical method transfer New descriptive approach for