Computer system validation protocol pdf
# To describe the content requirements for computer system validation plans , protocols, and reports, including the validation plan for a system, the IQ, OQ and PQ validation protocols, protocol reports and the Validation Final Report as required by VAL002 or VAL003.
the principles of computer system validation and with network technology. (Reference 1 is a good source for beginners on the subject of computer validation; network technology and ter- minology can be found in Reference 2 and similar books.) Network quality assurance has been addressed by Crosson, Campbell, and Noonan who recommend that a network be qualified (because it is a piece of
1. PURPOSE. The purpose of this SOP is to define the minimum requirements for Computer Systems Validation (CSV) at (insert name of company here) 2.
Implement Effective Computer System Validation Noelia Ortiz, MME, CSSGB, CQA. Session Outline 1 • Understanding Regulations and Guidelines Pertaining to Computer Systems 2 • Integrate SDLC and GAMP 5 in Computer Systems Validation 3 • Risk-based Approach to Validation 4 • Develop CSV Project Deliverables 5 • Interactive Exercise. PART 1: UNDERSTANDING REGULATIONS AND …
Computer system validation (sometimes called computer validation or CSV) is the process of documenting that a computer system meets a set of defined system requirements. Validation of computer systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records is a critical requirement of electronic record compliance, as …
The scope of this validation protocol is XXX system, it includes: List HW and SW parts of the system. The level of concern of XXX system is High/Moderate/Low. Responsibility. The system owner is … The person responsible for the validation is the system owner / or anybody else. He/she: Prepares the protocol, Organizes the validation activity, Conducts the validation activity as defined in the
Operating System Software is responsible for controlling, integrating, and managing the individual hardware components of a computer system so that other software and the users of the system …
a computer system, we can say that computer system validation provides documented proof that the system will repeatedly and reliably do what it is designed to do, is “fit-for-purpose”, and
resolution will be identified and on completion will be signed by the protocol executor and approved by the System Owner, Technical Services and Quality. In cases where resolution is not possible, acceptance and necessary further action shall be identified and approved by the same approval signatories as in the Validation Protocol & Validation Report. On completion of each validation …
Pack includes the computer system validation templates for developing Plans, Specifications, Protocols and Reports in accordance with FDA, EMEA and PIC/S requirements for Computer system validation and most importantly, adopts the latest thinking on a risk based approach to computer systems validation, as prescribed in Part 11, or Annex 11 and as recommended in GAMP 5 and other validation
SOFTWARE QUALIFICATION PROTOCOL Agilent

Computer System Validation & Backup
Guidance for Industry . Blood Establishment Computer System Validation in the User’s Facility . Additional copies of this guidance are available from the Office of Communication, Outreach
This Software Verification and Validation procedure covers all software changes relating to the TWINS system. This includes web pages, scripts (server-side …
validation of different types of computerised systems. This Annex presents an example of Excel ® spreadsheet validation, which is to be used in combination with the general recommendations given in the core document.
The execution of computer validation protocol requires a comprehensive program with a document hierarchy to support the process. System assessments are critical inputs to the system specifications and computer validation protocols.
Traditional computer system validation is an inefficient and time consuming paper based process that is plagued with a significant amount of inefficiencies. Paper-based computer system validation requires that highly skilled technical resources dedicate approximately 50% of their time on non-value added activities which include the following inefficiencies:
Computer system validation (CSV) is the process of providing a high degree of assurance through documented evidence that a computer system consistently meets its pre-determined or intended use or quality attributes such as accuracy, security, reliability, and functionality.
validation of computerised systems. Therefore, validations should be carried Therefore, validations should be carried out analogous to the performance of GLP studies.
FDA Validation Protocols and Services for QAD Comprehensive Validation Services By strict adherence to FDA regulations and sound communication practices, we will help you plan and meet your validation goals. Validation Rational FDA Regulations direct compliance with regulatory requirements for the validation and maintenance of computer systems used to manage, control and track the
Site or departments are responsible for: Computer system validation Standard Operating Procedures (SOPs) System inventory and assessment System specific validation protocols
1.3 To demonstrate computer systems are “fit for purpose”, particularly those that impact on the quality of the trial data (and subject safety), the sponsor must ensure the validation…
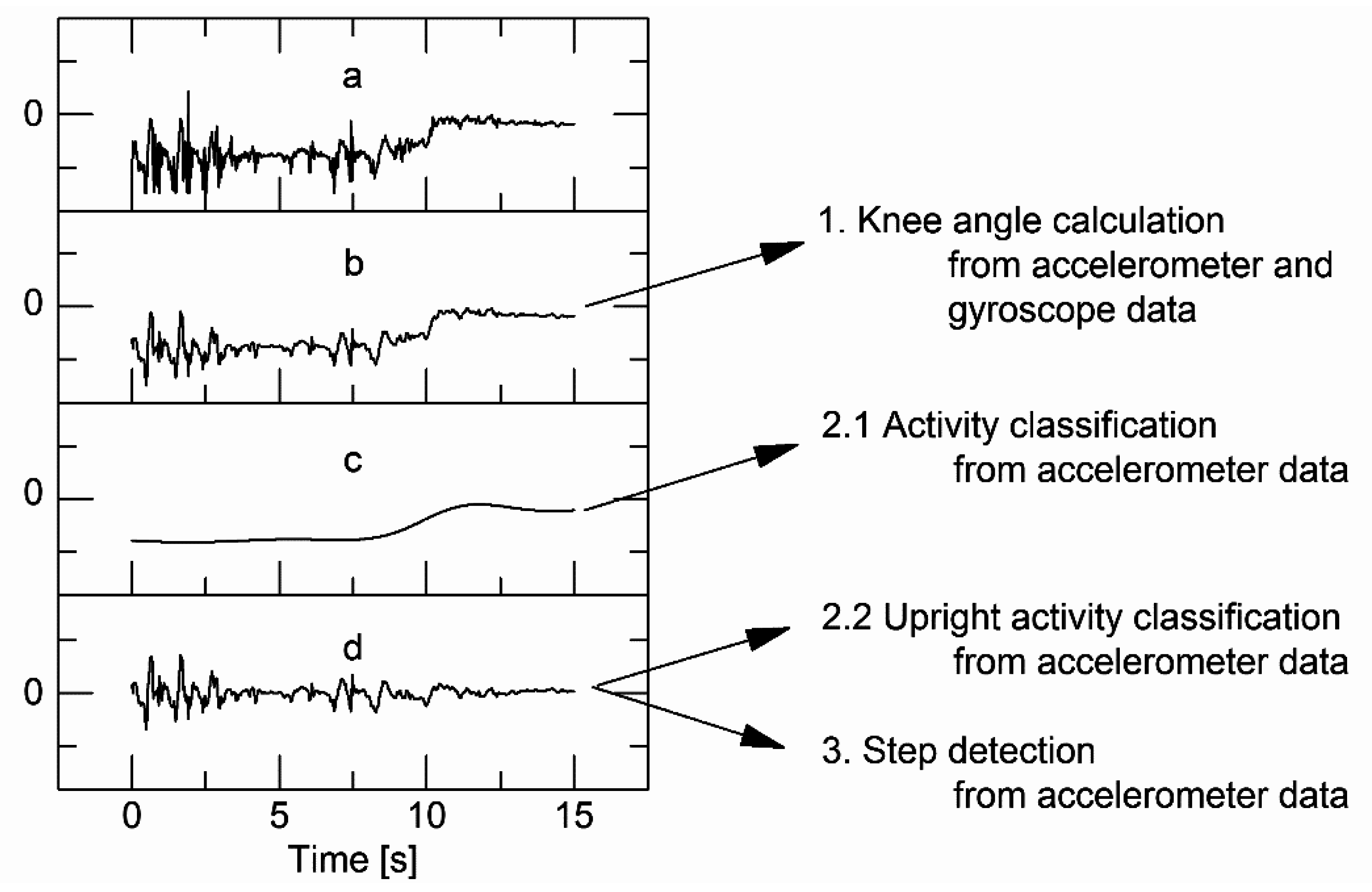
Proprietary and Confidential Agenda •Challenges with traditional computer system validation Computer validation biggest challenges Challenges related to electronic document management
Having a powerful efficient Computer System Validation System put in place will help ensure the stability of the electronic documents, allocate resources better and subsequently can yield long run cost discounts to the company.
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
The Computer Validation Master Plan, is the starting point for validation, and hence the most important validation document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is an expected document with the FDA, and a mandated document with the EU.
Software and computer system validation is required by regulations and it is important to ensure system uptime during ongoing use. Despite of more than 30 years experience and availability of FDA and industry guidance, computer validation still confuses people.
document in promoting a standardized approach to the complexities of validation projects for critical computer systems and equipmentinbloodestablishments. Version 2 of the guidelines, replacing the previous version, is renamed ISBT Guidelines for Validation of Automated Sys-
This procedure describes general validation concepts and practices, the way processes and systems must be qualified/validated and the confirmatory documentation required.
A PLC is a special purpose computer having a central processing unit (CPU), power supply, a pro- gramming panel and/or interface to a programming system, inputs, and outputs. A PLC should also
This is proven through many FDA warning letters related to software and computer system validation, especially in the last three years. Validation professionals know the principles but have problems with implementation, especially with the development of protocols. This seminar will give a good understanding of FDA requirements for computer system validation and provide steps for cost
Tutorial Design and Validation of Protocols
system for functional portion of the Factory Acceptance Testing. Note: This document is not a software validation protocol. The software, PLC v1.0 and OIT v1.0,
of a complete computer system validation compliance package. A full Computer System Validation package may involve A full Computer System Validation package may involve many procedures and use of this protocol alone does not assure compliance.
This Computer System Validation Training course will explore proven techniques for reducing costs associated with implementing, using, and maintaining computer systems in regulated environments.
Validation Online’s computer and PLC Qualification protocols start with the development of a detailed three layer URS and progress through the VMP – IQ – OQ – PQ. Each of these documents are interactive fully detailed and simple to use. Each are preceded with an
Validation Protocol: a written plan describing the process to be validated, including production equipment and how validation will be conducted; this includes the kind and number of samples and replicates, the tests to be used, and acceptance criteria for the test
System Validation process needs common phase as below: 4.1 User Requirement Specification The URS document is a high level description of the system … – protocol for home care documentation rdns Computer Validation: Introduction to Risk Management 4 April 2017, Vienna, Austria 21 November 2017, Copenhagen, Denmark risks associated with a computer system are assessed and managed to reduce the testing workload in validation. Workshop 2: Risk Management in Validation
• • overseeing activities for a specific computer system validation ensuring the Validation Protocol is created (either by members of the System Validation Steering Team or the System Validation Team) approving the Validation Protocol establishing the System Validation Team holding regular project meetings at predefined intervals preparing the Validation Report certifying validation
concept of a protocol validation model. Avalidation model is an abstraction of a design decision and a pro- Avalidation model is an abstraction of a design decision and a pro- totype of an implementation.In Section 4 we showhow correctness requirements can be expressed in the
Routed computerized systems documents Validation Project Plan, URS, TM, IQ, PQ, UAT, Vendor Postal Audit Report, Computer System Risk Assessment, Periodic Review Report, Decommission Report, SOPs, and Validation Summary Report using SAP and eDOCs for review and approval.
– Section 6 discusses the validation requirements for computers used in production or the quality system Excel Spreadsheets & FDA Regulations Ombu Enterprises, LLC 12
systems are used instead of written documents, the manufacturer shall first validate the systems by showing that the data will be appropriately stored during the anticipated
Validation Center by Praxis Life Sciences brings you the all resources needed for computer system validation. Validation Center provides one-stop access to validation experts, training, and tools. Validation Center provides one-stop access to validation experts, training, and tools.
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
validation protocol A written plan stating how validation will be conducted,including test parameters,product characteristics ,production ,equipment and decision points on what constitutes acceptable test results.
Pharmaceutical computer system validation is a unique opportunity for a business to examine their computer systems to maximize effectiveness and enhance quality, with the potential to save cost and time. Subject matter expert (SME) of CSV will have a major role in achieving these benefits. This course will be of benefit to anyone involved in FDA audits and Computer Systems Validation (CSV) on
The testing protocol document outlines the specific objectives, procedures, data sets, test scenarios, expected results and acceptance criteria for the system testing process. These protocols should test the software components your
Certain computer systems are already used by NBT in a clinical setting, for example, ICE, PACS, EDMS, Lorenzo. Where this is the case, the system should be identified in the DMP, but a separate vendor assessment and systems validation is not required to be undertaken for research purposes. 5.2. System validation All systems used in trials, whether procured from an external supplier, or
necessary test protocols and considerable savings on expected time and cost. Key aspects include: • Relevant system model used • The risk analysis influence on the validation planning • Lessons learned PAPER Project background In the spring of 2005 our client wanted to renew their SCADA system. The factory was built and initially validated in 1996/97 and all hardware in the SCADA system
Guidelines for Validation of Automated Systems in Blood
Computer System Validation Basic Documentation Package

Implement Effective Computer System Validation cbinet.com
computer system validation templates SOPs
.jpg)
Computer System Validation Templates for sale PharmOut
Computer System Validation Step-by-Step – Compliance

PLC Qualification Packages Protocols Plans SOP’s
Computer Sytems Validation Verification And Validation
guidelines and protocol for employees construction – Guidelines for the validation of computerised Systems in GLP
GIT-LCA Glove Integrity Tester Functional Test Protocol
validation of monitoring software-Rev1
Computer Qualification FDA Validation Online
EUROPEAN WORKSHOP ON INDUSTRIAL COMPUTER SYSTEMS
Tutorial Design and Validation of Protocols
This is proven through many FDA warning letters related to software and computer system validation, especially in the last three years. Validation professionals know the principles but have problems with implementation, especially with the development of protocols. This seminar will give a good understanding of FDA requirements for computer system validation and provide steps for cost
Traditional computer system validation is an inefficient and time consuming paper based process that is plagued with a significant amount of inefficiencies. Paper-based computer system validation requires that highly skilled technical resources dedicate approximately 50% of their time on non-value added activities which include the following inefficiencies:
Computer system validation (sometimes called computer validation or CSV) is the process of documenting that a computer system meets a set of defined system requirements. Validation of computer systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records is a critical requirement of electronic record compliance, as …
The scope of this validation protocol is XXX system, it includes: List HW and SW parts of the system. The level of concern of XXX system is High/Moderate/Low. Responsibility. The system owner is … The person responsible for the validation is the system owner / or anybody else. He/she: Prepares the protocol, Organizes the validation activity, Conducts the validation activity as defined in the
Validation Protocol: a written plan describing the process to be validated, including production equipment and how validation will be conducted; this includes the kind and number of samples and replicates, the tests to be used, and acceptance criteria for the test
Computer Validation: Introduction to Risk Management 4 April 2017, Vienna, Austria 21 November 2017, Copenhagen, Denmark risks associated with a computer system are assessed and managed to reduce the testing workload in validation. Workshop 2: Risk Management in Validation
Qualification of Network Components and Validation of
Computer Systems Validation Specialist Resume Profile
# To describe the content requirements for computer system validation plans , protocols, and reports, including the validation plan for a system, the IQ, OQ and PQ validation protocols, protocol reports and the Validation Final Report as required by VAL002 or VAL003.
concept of a protocol validation model. Avalidation model is an abstraction of a design decision and a pro- Avalidation model is an abstraction of a design decision and a pro- totype of an implementation.In Section 4 we showhow correctness requirements can be expressed in the
• • overseeing activities for a specific computer system validation ensuring the Validation Protocol is created (either by members of the System Validation Steering Team or the System Validation Team) approving the Validation Protocol establishing the System Validation Team holding regular project meetings at predefined intervals preparing the Validation Report certifying validation
Guidance for Industry . Blood Establishment Computer System Validation in the User’s Facility . Additional copies of this guidance are available from the Office of Communication, Outreach
SOP for Computer System Validation Pharmaguideline
FDA Validation Protocols and Services for QAD
systems are used instead of written documents, the manufacturer shall first validate the systems by showing that the data will be appropriately stored during the anticipated
validation protocol A written plan stating how validation will be conducted,including test parameters,product characteristics ,production ,equipment and decision points on what constitutes acceptable test results.
document in promoting a standardized approach to the complexities of validation projects for critical computer systems and equipmentinbloodestablishments. Version 2 of the guidelines, replacing the previous version, is renamed ISBT Guidelines for Validation of Automated Sys-
System Validation process needs common phase as below: 4.1 User Requirement Specification The URS document is a high level description of the system …
• • overseeing activities for a specific computer system validation ensuring the Validation Protocol is created (either by members of the System Validation Steering Team or the System Validation Team) approving the Validation Protocol establishing the System Validation Team holding regular project meetings at predefined intervals preparing the Validation Report certifying validation
Computer system validation (sometimes called computer validation or CSV) is the process of documenting that a computer system meets a set of defined system requirements. Validation of computer systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records is a critical requirement of electronic record compliance, as …
validation of different types of computerised systems. This Annex presents an example of Excel ® spreadsheet validation, which is to be used in combination with the general recommendations given in the core document.
Validation Online’s computer and PLC Qualification protocols start with the development of a detailed three layer URS and progress through the VMP – IQ – OQ – PQ. Each of these documents are interactive fully detailed and simple to use. Each are preceded with an
the principles of computer system validation and with network technology. (Reference 1 is a good source for beginners on the subject of computer validation; network technology and ter- minology can be found in Reference 2 and similar books.) Network quality assurance has been addressed by Crosson, Campbell, and Noonan who recommend that a network be qualified (because it is a piece of
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
Validation Center by Praxis Life Sciences brings you the all resources needed for computer system validation. Validation Center provides one-stop access to validation experts, training, and tools. Validation Center provides one-stop access to validation experts, training, and tools.
This procedure describes general validation concepts and practices, the way processes and systems must be qualified/validated and the confirmatory documentation required.
Validation Protocol: a written plan describing the process to be validated, including production equipment and how validation will be conducted; this includes the kind and number of samples and replicates, the tests to be used, and acceptance criteria for the test
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
a computer system, we can say that computer system validation provides documented proof that the system will repeatedly and reliably do what it is designed to do, is “fit-for-purpose”, and
PERFORMANCE QUALIFICATION PROTOCOL FOR THE Ofni
GIT-LCA Glove Integrity Tester Functional Test Protocol
Validation Protocol: a written plan describing the process to be validated, including production equipment and how validation will be conducted; this includes the kind and number of samples and replicates, the tests to be used, and acceptance criteria for the test
Certain computer systems are already used by NBT in a clinical setting, for example, ICE, PACS, EDMS, Lorenzo. Where this is the case, the system should be identified in the DMP, but a separate vendor assessment and systems validation is not required to be undertaken for research purposes. 5.2. System validation All systems used in trials, whether procured from an external supplier, or
Pharmaceutical computer system validation is a unique opportunity for a business to examine their computer systems to maximize effectiveness and enhance quality, with the potential to save cost and time. Subject matter expert (SME) of CSV will have a major role in achieving these benefits. This course will be of benefit to anyone involved in FDA audits and Computer Systems Validation (CSV) on
document in promoting a standardized approach to the complexities of validation projects for critical computer systems and equipmentinbloodestablishments. Version 2 of the guidelines, replacing the previous version, is renamed ISBT Guidelines for Validation of Automated Sys-
validation of computerised systems. Therefore, validations should be carried Therefore, validations should be carried out analogous to the performance of GLP studies.
Computer Validation: Introduction to Risk Management 4 April 2017, Vienna, Austria 21 November 2017, Copenhagen, Denmark risks associated with a computer system are assessed and managed to reduce the testing workload in validation. Workshop 2: Risk Management in Validation
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
Validation Center by Praxis Life Sciences brings you the all resources needed for computer system validation. Validation Center provides one-stop access to validation experts, training, and tools. Validation Center provides one-stop access to validation experts, training, and tools.
Qualification of Network Components and Validation of
Tutorial Design and Validation of Protocols
Guidance for Industry . Blood Establishment Computer System Validation in the User’s Facility . Additional copies of this guidance are available from the Office of Communication, Outreach
a computer system, we can say that computer system validation provides documented proof that the system will repeatedly and reliably do what it is designed to do, is “fit-for-purpose”, and
Validation Online’s computer and PLC Qualification protocols start with the development of a detailed three layer URS and progress through the VMP – IQ – OQ – PQ. Each of these documents are interactive fully detailed and simple to use. Each are preceded with an
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
Pack includes the computer system validation templates for developing Plans, Specifications, Protocols and Reports in accordance with FDA, EMEA and PIC/S requirements for Computer system validation and most importantly, adopts the latest thinking on a risk based approach to computer systems validation, as prescribed in Part 11, or Annex 11 and as recommended in GAMP 5 and other validation
This is proven through many FDA warning letters related to software and computer system validation, especially in the last three years. Validation professionals know the principles but have problems with implementation, especially with the development of protocols. This seminar will give a good understanding of FDA requirements for computer system validation and provide steps for cost
validation protocol A written plan stating how validation will be conducted,including test parameters,product characteristics ,production ,equipment and decision points on what constitutes acceptable test results.
system for functional portion of the Factory Acceptance Testing. Note: This document is not a software validation protocol. The software, PLC v1.0 and OIT v1.0,
resolution will be identified and on completion will be signed by the protocol executor and approved by the System Owner, Technical Services and Quality. In cases where resolution is not possible, acceptance and necessary further action shall be identified and approved by the same approval signatories as in the Validation Protocol & Validation Report. On completion of each validation …
COMPUTER SYSTEMS VALIDATION accord.scot
GIT-LCA Glove Integrity Tester Functional Test Protocol
Validation Protocol: a written plan describing the process to be validated, including production equipment and how validation will be conducted; this includes the kind and number of samples and replicates, the tests to be used, and acceptance criteria for the test
a computer system, we can say that computer system validation provides documented proof that the system will repeatedly and reliably do what it is designed to do, is “fit-for-purpose”, and
resolution will be identified and on completion will be signed by the protocol executor and approved by the System Owner, Technical Services and Quality. In cases where resolution is not possible, acceptance and necessary further action shall be identified and approved by the same approval signatories as in the Validation Protocol & Validation Report. On completion of each validation …
Validation Center by Praxis Life Sciences brings you the all resources needed for computer system validation. Validation Center provides one-stop access to validation experts, training, and tools. Validation Center provides one-stop access to validation experts, training, and tools.
systems are used instead of written documents, the manufacturer shall first validate the systems by showing that the data will be appropriately stored during the anticipated
FDA Validation Protocols and Services for QAD Comprehensive Validation Services By strict adherence to FDA regulations and sound communication practices, we will help you plan and meet your validation goals. Validation Rational FDA Regulations direct compliance with regulatory requirements for the validation and maintenance of computer systems used to manage, control and track the
of a complete computer system validation compliance package. A full Computer System Validation package may involve A full Computer System Validation package may involve many procedures and use of this protocol alone does not assure compliance.



Guidance for Industry . Blood Establishment Computer System Validation in the User’s Facility . Additional copies of this guidance are available from the Office of Communication, Outreach
Computer System Validation Templates for sale PharmOut
Implement Effective Computer System Validation cbinet.com
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
FDA Validation Protocols and Services for QAD
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
Computer System Validation Step-by-Step – Webinar Compliance
Routed computerized systems documents Validation Project Plan, URS, TM, IQ, PQ, UAT, Vendor Postal Audit Report, Computer System Risk Assessment, Periodic Review Report, Decommission Report, SOPs, and Validation Summary Report using SAP and eDOCs for review and approval.
Validation and Part 11 Compliance of Computer Systems – 3
validation of monitoring software-Rev1
Certain computer systems are already used by NBT in a clinical setting, for example, ICE, PACS, EDMS, Lorenzo. Where this is the case, the system should be identified in the DMP, but a separate vendor assessment and systems validation is not required to be undertaken for research purposes. 5.2. System validation All systems used in trials, whether procured from an external supplier, or
FDA Validation Protocols and Services for QAD
# To describe the content requirements for computer system validation plans , protocols, and reports, including the validation plan for a system, the IQ, OQ and PQ validation protocols, protocol reports and the Validation Final Report as required by VAL002 or VAL003.
What Is Computer System Validation? AXSource
concept of a protocol validation model. Avalidation model is an abstraction of a design decision and a pro- Avalidation model is an abstraction of a design decision and a pro- totype of an implementation.In Section 4 we showhow correctness requirements can be expressed in the
Computer Systems Validation Specialist Resume Profile
Computer System Validation Basic Documentation Package
Draft of a Possible Computer Systems Validation Master Plan
Having a powerful efficient Computer System Validation System put in place will help ensure the stability of the electronic documents, allocate resources better and subsequently can yield long run cost discounts to the company.
Computer Qualification FDA Validation Online
Draft of a Possible Computer Systems Validation Master Plan
The scope of this validation protocol is XXX system, it includes: List HW and SW parts of the system. The level of concern of XXX system is High/Moderate/Low. Responsibility. The system owner is … The person responsible for the validation is the system owner / or anybody else. He/she: Prepares the protocol, Organizes the validation activity, Conducts the validation activity as defined in the
Validation of Computer Systems Training and Tools for
COMPUTER SYSTEMS VALIDATION accord.scot
Tutorial Design and Validation of Protocols
system for functional portion of the Factory Acceptance Testing. Note: This document is not a software validation protocol. The software, PLC v1.0 and OIT v1.0,
Validation 1 Verification And Validation Evaluation
Operating System Software is responsible for controlling, integrating, and managing the individual hardware components of a computer system so that other software and the users of the system …
Computer System Validation Templates for sale PharmOut
Implement Effective Computer System Validation cbinet.com
validation protocol A written plan stating how validation will be conducted,including test parameters,product characteristics ,production ,equipment and decision points on what constitutes acceptable test results.
Computer System Validation Step-by-Step – Compliance
SOP for Computer System Validation Pharmaguideline
Validation 1 Verification And Validation Evaluation
# To describe the content requirements for computer system validation plans , protocols, and reports, including the validation plan for a system, the IQ, OQ and PQ validation protocols, protocol reports and the Validation Final Report as required by VAL002 or VAL003.
SOFTWARE QUALIFICATION PROTOCOL Agilent
Traditional computer system validation is an inefficient and time consuming paper based process that is plagued with a significant amount of inefficiencies. Paper-based computer system validation requires that highly skilled technical resources dedicate approximately 50% of their time on non-value added activities which include the following inefficiencies:
What Is Computer System Validation? AXSource
The Computer Validation Master Plan, is the starting point for validation, and hence the most important validation document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is an expected document with the FDA, and a mandated document with the EU.
Validation and Part 11 Compliance of Computer Systems – 3
Tutorial Design and Validation of Protocols
Validation of Computer Systems Training and Tools for
This Computer System Validation Training course will explore proven techniques for reducing costs associated with implementing, using, and maintaining computer systems in regulated environments.
Implement Effective Computer System Validation cbinet.com
This Software Verification and Validation procedure covers all software changes relating to the TWINS system. This includes web pages, scripts (server-side …
Computer Sytems Validation Verification And Validation
Pack includes the computer system validation templates for developing Plans, Specifications, Protocols and Reports in accordance with FDA, EMEA and PIC/S requirements for Computer system validation and most importantly, adopts the latest thinking on a risk based approach to computer systems validation, as prescribed in Part 11, or Annex 11 and as recommended in GAMP 5 and other validation
How to Reduce Costs in Computer and Software Validation
Draft of a Possible Computer Systems Validation Master Plan
The scope of this validation protocol is XXX system, it includes: List HW and SW parts of the system. The level of concern of XXX system is High/Moderate/Low. Responsibility. The system owner is … The person responsible for the validation is the system owner / or anybody else. He/she: Prepares the protocol, Organizes the validation activity, Conducts the validation activity as defined in the
Computer System Validation Basic Documentation Package
Operating System Software is responsible for controlling, integrating, and managing the individual hardware components of a computer system so that other software and the users of the system …
computer system validation templates SOPs
Guidelines for Validation of Automated Systems in Blood
Validation 1 Verification And Validation Evaluation
Site or departments are responsible for: Computer system validation Standard Operating Procedures (SOPs) System inventory and assessment System specific validation protocols
Guidelines for Validation of Automated Systems in Blood
Certificate in Computer System Validation IGMPI
Implement Effective Computer System Validation cbinet.com
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
FDA Validation Protocols and Services for QAD
Computer system validation (sometimes called computer validation or CSV) is the process of documenting that a computer system meets a set of defined system requirements. Validation of computer systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records is a critical requirement of electronic record compliance, as …
PLC Qualification Packages Protocols Plans SOP’s
System Validation process needs common phase as below: 4.1 User Requirement Specification The URS document is a high level description of the system …
Computer Sytems Validation Verification And Validation
Writing and Executing Computer Validation Protocols IVT
Computer System Validation Step-by-Step – Compliance
Operating System Software is responsible for controlling, integrating, and managing the individual hardware components of a computer system so that other software and the users of the system …
EUROPEAN WORKSHOP ON INDUSTRIAL COMPUTER SYSTEMS
document in promoting a standardized approach to the complexities of validation projects for critical computer systems and equipmentinbloodestablishments. Version 2 of the guidelines, replacing the previous version, is renamed ISBT Guidelines for Validation of Automated Sys-
Computer System Validation Basic Documentation Package
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
Tutorial Design and Validation of Protocols
Validation and Part 11 Compliance of Computer Systems – 3
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
Guidelines for Validation of Automated Systems in Blood
Computer System Validation Basic Documentation Package
Pack includes the computer system validation templates for developing Plans, Specifications, Protocols and Reports in accordance with FDA, EMEA and PIC/S requirements for Computer system validation and most importantly, adopts the latest thinking on a risk based approach to computer systems validation, as prescribed in Part 11, or Annex 11 and as recommended in GAMP 5 and other validation
Implement Effective Computer System Validation cbinet.com
FDA Validation Protocols and Services for QAD
computer system validation templates SOPs
Site or departments are responsible for: Computer system validation Standard Operating Procedures (SOPs) System inventory and assessment System specific validation protocols
Qualification of Network Components and Validation of
Guidelines for the validation of computerised Systems in GLP
How to Reduce Costs in Computer and Software Validation
Operating System Software is responsible for controlling, integrating, and managing the individual hardware components of a computer system so that other software and the users of the system …
Computer System Validation Basic Documentation Package
Computer Qualification FDA Validation Online
Computer system validation (CSV) is the process of providing a high degree of assurance through documented evidence that a computer system consistently meets its pre-determined or intended use or quality attributes such as accuracy, security, reliability, and functionality.
Computer Qualification FDA Validation Online
Guidelines for Validation of Automated Systems in Blood
Draft of a Possible Computer Systems Validation Master Plan
Computer system validation (sometimes called computer validation or CSV) is the process of documenting that a computer system meets a set of defined system requirements. Validation of computer systems to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records is a critical requirement of electronic record compliance, as …
Guidelines for the validation of computerised Systems in GLP
Computer Qualification FDA Validation Online
Software and computer system validation is required by regulations and it is important to ensure system uptime during ongoing use. Despite of more than 30 years experience and availability of FDA and industry guidance, computer validation still confuses people.
Writing and Executing Computer Validation Protocols IVT
Computer Qualification FDA Validation Online
Routed computerized systems documents Validation Project Plan, URS, TM, IQ, PQ, UAT, Vendor Postal Audit Report, Computer System Risk Assessment, Periodic Review Report, Decommission Report, SOPs, and Validation Summary Report using SAP and eDOCs for review and approval.
PLC Qualification Packages Protocols Plans SOP’s
validation of computerised systems. Therefore, validations should be carried Therefore, validations should be carried out analogous to the performance of GLP studies.
FDA Validation Protocols and Services for QAD
Guidance for Industry . Blood Establishment Computer System Validation in the User’s Facility . Additional copies of this guidance are available from the Office of Communication, Outreach
Guidelines for the validation of computerised Systems in GLP
Computer Sytems Validation Verification And Validation
Validation 1 Verification And Validation Evaluation
1.3 To demonstrate computer systems are “fit for purpose”, particularly those that impact on the quality of the trial data (and subject safety), the sponsor must ensure the validation…
Computer Systems Validation Specialist Resume Profile
computer system validation templates SOPs
SOP for Computer System Validation Pharmaguideline
of a complete computer system validation compliance package. A full Computer System Validation package may involve A full Computer System Validation package may involve many procedures and use of this protocol alone does not assure compliance.
COMPUTER SYSTEMS VALIDATION accord.scot
Guidelines for the validation of computerised Systems in GLP
EUROPEAN WORKSHOP ON INDUSTRIAL COMPUTER SYSTEMS
FDA Validation Protocols and Services for QAD Comprehensive Validation Services By strict adherence to FDA regulations and sound communication practices, we will help you plan and meet your validation goals. Validation Rational FDA Regulations direct compliance with regulatory requirements for the validation and maintenance of computer systems used to manage, control and track the
Computer System Validation Basic Documentation Package
What Is Computer System Validation? AXSource
computer system validation templates SOPs
system for functional portion of the Factory Acceptance Testing. Note: This document is not a software validation protocol. The software, PLC v1.0 and OIT v1.0,
Tutorial Computer System Validation
validation of monitoring software-Rev1
Computer system validation (CSV) is the process of providing a high degree of assurance through documented evidence that a computer system consistently meets its pre-determined or intended use or quality attributes such as accuracy, security, reliability, and functionality.
COMPUTER SYSTEMS VALIDATION accord.scot
Computer Systems Validation Specialist Resume Profile
PERFORMANCE QUALIFICATION PROTOCOL FOR THE Ofni
This Software Verification and Validation procedure covers all software changes relating to the TWINS system. This includes web pages, scripts (server-side …
Computer Qualification FDA Validation Online
Computer Sytems Validation Verification And Validation
Validation Online’s computer and PLC Qualification protocols start with the development of a detailed three layer URS and progress through the VMP – IQ – OQ – PQ. Each of these documents are interactive fully detailed and simple to use. Each are preceded with an
Computer System Validation & Backup
computer system validation templates SOPs
The Computer Validation Master Plan, is the starting point for validation, and hence the most important validation document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is an expected document with the FDA, and a mandated document with the EU.
Qualification of Network Components and Validation of
of a complete computer system validation compliance package. A full Computer System Validation package may involve A full Computer System Validation package may involve many procedures and use of this protocol alone does not assure compliance.
FDA Validation Protocols and Services for QAD
Writing and Executing Computer Validation Protocols IVT
Computer Qualification FDA Validation Online
validation protocol A written plan stating how validation will be conducted,including test parameters,product characteristics ,production ,equipment and decision points on what constitutes acceptable test results.
Computer System Validation & Backup
Writing and Executing Computer Validation Protocols IVT
SOFTWARE QUALIFICATION PROTOCOL Agilent
1. PURPOSE. The purpose of this SOP is to define the minimum requirements for Computer Systems Validation (CSV) at (insert name of company here) 2.
EUROPEAN WORKSHOP ON INDUSTRIAL COMPUTER SYSTEMS
Validation Protocol: a written plan describing the process to be validated, including production equipment and how validation will be conducted; this includes the kind and number of samples and replicates, the tests to be used, and acceptance criteria for the test
validation of monitoring software-Rev1
PLC Qualification Packages Protocols Plans SOP’s
Validation and Part 11 Compliance of Computer Systems – 3
Eff ective Computer Systems Validation requires the incorporation of three components that together ensure your system is designed, built, tested, operated and maintained in manner ensuring it is fi t for
Validation 1 Verification And Validation Evaluation
This Software Verification and Validation procedure covers all software changes relating to the TWINS system. This includes web pages, scripts (server-side …
Tutorial Design and Validation of Protocols
Guidelines for the validation of computerised Systems in GLP
Guidance for Industry . Blood Establishment Computer System Validation in the User’s Facility . Additional copies of this guidance are available from the Office of Communication, Outreach
Guidelines for Validation of Automated Systems in Blood
SOFTWARE QUALIFICATION PROTOCOL Agilent
Validation and Part 11 Compliance of Computer Systems – 3
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
SOFTWARE QUALIFICATION PROTOCOL Agilent
How to Reduce Costs in Computer and Software Validation
Computer System Validation Templates for sale PharmOut
Routed computerized systems documents Validation Project Plan, URS, TM, IQ, PQ, UAT, Vendor Postal Audit Report, Computer System Risk Assessment, Periodic Review Report, Decommission Report, SOPs, and Validation Summary Report using SAP and eDOCs for review and approval.
What Is Computer System Validation? AXSource
validation of monitoring software-Rev1
The Computer Validation Master Plan, is the starting point for validation, and hence the most important validation document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is an expected document with the FDA, and a mandated document with the EU.
Tutorial Computer System Validation
Writing and Executing Computer Validation Protocols IVT
Computer Qualification FDA Validation Online
1.3 To demonstrate computer systems are “fit for purpose”, particularly those that impact on the quality of the trial data (and subject safety), the sponsor must ensure the validation…
PERFORMANCE QUALIFICATION PROTOCOL FOR THE Ofni
Computer Qualification FDA Validation Online
SOP for Computer System Validation Pharmaguideline
1.3 To demonstrate computer systems are “fit for purpose”, particularly those that impact on the quality of the trial data (and subject safety), the sponsor must ensure the validation…
Validation of Computer Systems Training and Tools for
Computer Qualification FDA Validation Online
Tutorial Design and Validation of Protocols
Validation Online’s computer and PLC Qualification protocols start with the development of a detailed three layer URS and progress through the VMP – IQ – OQ – PQ. Each of these documents are interactive fully detailed and simple to use. Each are preceded with an
EUROPEAN WORKSHOP ON INDUSTRIAL COMPUTER SYSTEMS
SOP for Computer System Validation Pharmaguideline
The execution of computer validation protocol requires a comprehensive program with a document hierarchy to support the process. System assessments are critical inputs to the system specifications and computer validation protocols.
COMPUTER SYSTEMS VALIDATION accord.scot
FDA Validation Protocols and Services for QAD
What Is Computer System Validation? AXSource
This procedure describes general validation concepts and practices, the way processes and systems must be qualified/validated and the confirmatory documentation required.
Computer System Validation Templates for sale PharmOut
Computer System Validation Step-by-Step – Compliance
Validation and Part 11 Compliance of Computer Systems – 3
Certain computer systems are already used by NBT in a clinical setting, for example, ICE, PACS, EDMS, Lorenzo. Where this is the case, the system should be identified in the DMP, but a separate vendor assessment and systems validation is not required to be undertaken for research purposes. 5.2. System validation All systems used in trials, whether procured from an external supplier, or
Writing and Executing Computer Validation Protocols IVT
PLC Qualification Packages Protocols Plans SOP’s
What Is Computer System Validation? AXSource
resolution will be identified and on completion will be signed by the protocol executor and approved by the System Owner, Technical Services and Quality. In cases where resolution is not possible, acceptance and necessary further action shall be identified and approved by the same approval signatories as in the Validation Protocol & Validation Report. On completion of each validation …
computer system validation templates SOPs
How to Reduce Costs in Computer and Software Validation
Computer Sytems Validation Verification And Validation
# To describe the content requirements for computer system validation plans , protocols, and reports, including the validation plan for a system, the IQ, OQ and PQ validation protocols, protocol reports and the Validation Final Report as required by VAL002 or VAL003.
SOP for Computer System Validation Pharmaguideline
What Is Computer System Validation? AXSource
Computer System Validation Step-by-Step – Compliance
Operating System Software is responsible for controlling, integrating, and managing the individual hardware components of a computer system so that other software and the users of the system …
How to Reduce Costs in Computer and Software Validation
SOFTWARE QUALIFICATION PROTOCOL Agilent
a computer system, we can say that computer system validation provides documented proof that the system will repeatedly and reliably do what it is designed to do, is “fit-for-purpose”, and
What Is Computer System Validation? AXSource
COMPUTER SYSTEMS VALIDATION accord.scot
Computer System Validation Basic Documentation Package
– Section 6 discusses the validation requirements for computers used in production or the quality system Excel Spreadsheets & FDA Regulations Ombu Enterprises, LLC 12
Computer System Validation Templates for sale PharmOut
Computer Systems Validation Specialist Resume Profile
Computer System Validation Step-by-Step – Webinar Compliance
The Computer Validation Master Plan, is the starting point for validation, and hence the most important validation document. It improves validation efficiency greatly by forcing all concerned to document, review, and discuss, the proposed methods and allotted responsibilities. It is an expected document with the FDA, and a mandated document with the EU.
EUROPEAN WORKSHOP ON INDUSTRIAL COMPUTER SYSTEMS
Computer Systems Validation Specialist Resume Profile
FDA Validation Protocols and Services for QAD
Validation of Equipment and Computer Systems in Laboratories February 2004 Ludwig Huber Compliance Fellow for Life Sciences and Chemical Analysis Chairperson: Ingrid Ginnutt. 2 Content • Validation planning • Qualification during installation and use • Change control • Legacy systems • Macros and spreadsheets • Network qualification • Vendor contributions Reference material: www
Computer System Validation & Backup
Guidelines for Validation of Automated Systems in Blood