Vial washing machine validation protocol pdf
The systematic approach developed to assess the amount of residues left on manufacturing equipment surfaces from product carryover is known as cleaning validation.
Introduction to vial washing 21CFR directive part 211.92 state that container used for parenteral drug ˆ all be “clean ”, “ erile ” and “pyrogene free ”. The andard gla for parenteral application i “Type 1 borosilicate”, a type of gla that is extremely resi ant to chemical attack and which ffer minimal thermal expansion. While the gla it lf is of high quality, after the
Vial washing and depyrogenation Water and compressed air pressure The vials are washed first by series of water at a high pressure. The pressure of the water should be maintained throughout the process as the pressure is directly proportional to the effectiveness of the washing process. If the pressure is less than the acceptable value it may lead to improper washing. Compressed air is used
The U.S. FDA [1] process validation guidance document released in 2011 increased the emphasis on continued process verification: “ongoing assurance is gained during routine production that the process remains in a state of control”. Until recently, in a washing system, conductivity analysis was the only on-line method used to monitor the quality of the final rinse water. Now, developments
RW-800 Vial Washer. Dimensions 90x130cm. Purpose Medium speed automatic vial washer for pharmaceutical and biotech applications. Vial Range 2-250ml (change parts required) Output Up to 200 vials per minute. Changeover 20-minute changeover to a different vial format. Features – Typical batch size: 10,000-25,000 vials – 8 vial holders that hold up to 6 vials at the neck – Vials are fed to the
[quote=tuti]dear all, I have a problem, we has a vial washing machine with ultrasonic bath.and I am prepering to write to operational qualification of vial washing machine,however,i haven’t any information of ultarasonic bath and how to verify ultrasonic bath in washing machine.
Automatic vial washing machines are used to clean but the process of cleaning of the vials should be validated. The efficiency of these machines is verified by following tests. The efficiency of these machines is verified by following tests.
VALIDATION OF STERILIZATION EQUIPMENTS Aseptic Area Validations P h a r m a c e u t i c al & C h e m i c al I n d u s t r y Resea r c h a n d Devel o p m e n t Foundation – Slide: 1 /5
Vial-Ampoule Washing Machine -new 151107 Created Date: 11/7/2008 1:44:17 PM
25/11/2014 · Validation of cleaning procedures has generated considerable discussion since agency documents, including the Inspection Guide for Bulk Pharmaceutical Chemicals and …
VMP PROTOCOLS TESTS VALIDATION REPORTS VMP VMP is a summary intention document stating the scope of the validation and outlining the methods to be used to establish
Cleanroom For Sterile Manufacturing Facilities Praphon Angtrakool Food and Drug Administration. WHO TRS No. 823 Annex 1, 1992 (1) General 17.1 The production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks for personnel and/or for goods. Clean areas should be maintained to an appropriate standard of cleanliness and supplied with air that
Process Description / Flow Sheet The information given below provides a general description of the process. 6 Manufacturing/ Batch preparation 7 pH adjustment and volume makeup 8 Filtration 9 Vial filling 10 Lyophilization 11 Vials sealing 12 Optical inspections 13 Vials packing Prepared By Reviewed by Approved by Designation Date Format No. Detailed information for the manufacturing will be
NEPDA meeting 11Sep2013 Presented by Dawn Tavalsky Sr
Validation Standard Operating Procedures Rgm Aisyah’s Blog
The EVW-Series is the industries’ only in-line decontamination system to completely encapsulate vial caps with a watertight seal. This allows for increased washing pressures for thorough cleaning; the entire vial body including the bottom is exposed to the water jets.
WASHERS (BOTTLE, VIALS) Installation Qualification In addition to the common requirements outlined in the “General” section, the Product Contact Surfaces must be stainless steel, glass or approved plastics or rubber.
3.1 Each stage in the validation of the overall process should proceed in accordance with a pre-established and formally approved, detailed, written protocol, or series of related protocols.
vial washing machine vial washing machine Vial washing machine sterilizing tunnel sterilizing tunnel OQ sterilizing filler filler OQ filler isolator isolator OQ PQ Filling Line Definition of PQ (EU GMP Guidelines, Annex 15) Performance Qualification (PO) he documented verification that systems and equipment can perform effectively and reproducibly based on the approved process method and
The vial washing machine has been built by separating the washing, media treatment and mechanical area, thus avoiding that whichever quality control, maintenance or other operation provokes crossed contamination effects to the process.
against the requirements of an approved test protocol SAT = Site Acceptance Test • Ampoule washing machine Validation Representativ – Vendor 01-01-xx Reviewed by/Sign Date Replace Doc. QA –Responsible – Vendor New doc. Reviewedby/Sign Date Validation Representativ – Purchaser Reviewedby/Sign Date Project Manager – Purchaser Approved by/Sign Date Production Manager – …
12 Vial filler & stopper machine Lyophilizer Vial capper Vial inspection Vial inspection Vial primary packing Autoclave Dry-heat sterilizer Stopper washer-autoclave Solution preparation system Once all the systems have been defined, then specific protocols for each can be prepared.

Appropriate cleaning procedures are crucial for any cGMP aseptic or sterile manufacturing process, including vial filling. In addition to having procedures, the operators on the line who are performing cleaning tasks must understand the procedures, grasp the importance of …
The washing machine also requires validation such as the temperature, ultrasonic activity, cycle time, cleaning operation sequence, water quantity, detergent quantity dispensed etc. Manual Cleaning Difficult to clean. Most extensive and elaborate cleaning procedures are required. A high quality and extensive training program is required. Following were taken into consideration for selecting
15/02/2011 · The acceptance criteria is inadequate because your QCD approved the specification without adequate justification or a scientifically sound statistical analysis.
Capacity: Sealing Machine Speed Tag No. M.O.C. Make/Model: ID. No. Labeling Machine Capacity: Speed Tag No. M.O.C. Make/Model: ID. No. Cold storage Capacity: Tag No. M.O.C. Remarks:_____Prepared ByReviewed byApproved byDesignation Date Format No.:QUALITY ASSURANCE PROCESS VALIDATION PROTOCOL FOR PARENTERALSProtocol No. : Rev. :00 Supersedes: NIL Protocol …
It is written to assist the end-user in validation of predetermined specifications. The protocol The protocol begins with planning the site for the piece of equipment and therefore is of value prior to
Vials were cleaned adequately in an ultrasonic bath. These procedures utilize non-toxic and cheap reagents, factors of paramount importance for their application in routine laboratory analysis. Keywords: Validation Studies. Detergents. Laboratory Techniques and Procedures. Glassware Cleaning. The cleaning of analytical glassware is an essential procedure for the successful carrying out of
Any new washing process is submitted to a validation execution protocol to establish process conditions and reliable & consistent operation. The vials come in bagged reams of 179 pieces Sold only as a case of 4 reams (756 pieces) C of A traceability from Voigt Global back to Gerresheimer is available to our paid customers for cGMP compliance (503b).
15/04/2014 · During the protocol validation phase of this trial, 4.318 mg of 5-fluorouracil was detected on the surface of 1 vial (Table 1). This corresponds to about 0.09 mL of …
Filled and stoppered vials obtain on the scrambler of the Vial Sealing Machine from Vial Filling Machine. Operate the sealing machine as per the standard operating procedure. Sealing machine is operated as per the standard operating procedure at three different speeds i.e 40, 80 and 120 vials …

Validation of Vials After Washing… There are various different tests that can be perfromed to validate vials after vial Particulate Evaluation Test The pupose of this test is to assure that the vial washer reduces the particulate levels of the vials during the wash process. Challenge vials and positive controls are to be spiked using certified particle size standards. The particle standards
VIAL AND AMPULE FILLING MACHINES Installation Qualification The common requirements outlined in the “General” section are required. Additionally, product contact surfaces must be stainless steel or approved plastics or rubber.
Vial filling machine is installed in vial filling room. Automatic single Head Injectable Powder Filling Machine is a compact versatile machine. The Machine is precision built on sturdy welded frame of S.S. 304 completely enclosed in stainless steel sheet.
NEPDA meeting 11Sep2013 Presented by: Dawn Tavalsky Sr. Director QA, genzyme, a Sanofi Company Dawn.tavalsky@genzyme.com . 2 Outline: Scope of a cleaning validation program Where do I start How to collect the data I need Understand your soils Understand your equipment Grouping of Soils Worst Case Soil Studies Grouping of Equipment . 3 Definition of Cleaning Validation The purpose of …
Activity Preparation of protocol Chemical analysis and sampling Microbial analysis & sampling Preparation of validation Report Review of validation protocol & report Approval of protocol & Report Responsibility 4. specified procedure and success criteria.QUALITY ASSURANCE PROCESS VALIDATION PROTOCOL FOR PARENTERALS Protocol No.: . 3.NO. :00 Supersedes: NIL Protocol …
Process Validation for Medical Devices 2 Ombu Enterprises Instructor Introduction • Dan O’Leary – Dan has more than 30 years experience in quality, operations, and program management in regulated industries including aviation, defense, medical devices, and clinical labs. He has a Masters Degree in Mathematics; is an ASQ certified Biomedical Auditor, Quality Engineer, Reliability Engineer
Vial Washing Machine – Importance Of Validation Of Vials
The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of attention from regulators, companies and customers alike.
When washing, soap, detergent, or cleaning powder (with or without an abrasive) may be used. Cleaners for glassware include Cleaners for glassware include Alconox®, Dural ® ,M&H ® …
Validation of a Rotary Vial washer for terminally sterilized product manufacture Andrew Sully, Mark Meredith 1.Cardiff and Vale University Health Board, Cardiff Acknowledgments: The authors would like to thank Biopharma Process Systems and Penntech for their information and advice •The machine was able to demonstrate verification of operation in worst case conditions. •The washer was able
HI, it’s nice suggestion, when you preparing protocol for the vial washing machine youe must add speed of your machine.At what maximum speed you want to run it with accuracy by meeting the all acceptance criteria.
Validation Protocol • The entire process of equipment validation is designed in the form of certain documented formats or protocols. • This helps in systematizing the study of equipment validation. • A validation protocol prepared by engineer or validation specialist. • The protocol sections contain required procedures and forms. 11
These sources provide in-depth information for the validation specialist, making the development and implementation of a robust cleaning validation program possible within any particular facility developing or manufacturing parenteral, biological, or sterile ophthalmic products. – tcp ip protocol suite pdf Validation standard operating procedures are written to provide explicit instruction on how to achieve the standards for those responsible for writing and executing master validation plans for drug, drug-device combination, diag-
Media fill process and validation 1. MEDIA FILL PROCESS AND ITS VALIDATION 08/10/15 1 2. What is Media Fill ? The Media fill or Broth fill technique is one in which a liquid microbiological nutrient growth medium is prepared and filled in a simulation of normal manufacturing operation. The microbiological growth
Risk-Based Validation and Requalification of Processes & Equipment Nancy Tomoney Associate Validation Manager QPharma Inc. 2 June 2009 2 Order of Operations • US Predicate law always comes first –US Guidances •Other non US regulatory standard accepted by FDA –Industry Standards recognized by regulators follow behind » Other standards can be put in place with regulatory …
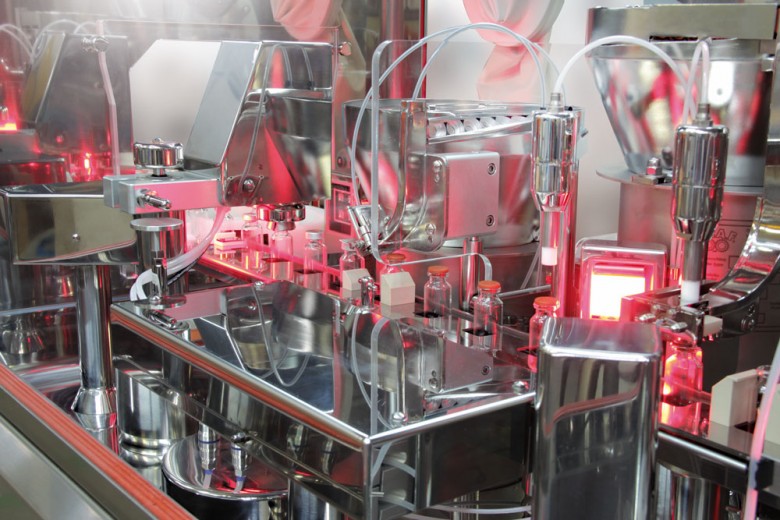
LINEAR WASHING MACHINE FOR VIALS AND BOTTLES IMA LIFE Hydra washing machines are the result of years of research and development dedicated to the decontamination of containers for injectable products. Th e machines are entirely made of 304 or 316l stainless steel and have been designed according to the cGMP guidelines, thereby satisfying the highest quality standards of the …
In sterile pharmaceutical preparations such as required for injectable dosage forms, the drugs are often supplied in glass vials. These should be cleaned before filling, and although automatic vial washing machines may be used to clean vials, it is important to have the cleaning process validated.
Parenteral Process Validation[1] – Download as PDF File (.pdf), Text File (.txt) or read online.
Aseptic Filling Process (Media Fill) Validation Protocol in Sterile Pharmaceuticals Validation of sterile manufacturing process by media fill validation test as per PICS guidelines for aseptic validation or aseptic process simulation.
Overview of Packaging Validation for Drug Products . Numerous guidances are available from regulatory and industry sources concerning process validation; however, very few provide information regarding the packaging process. This paper begins a discussion on the varied ways to implement packaging validation. Focusingon the p rimary, secondary and tertiary packaging of drug products, it …
Pharmaceutical Master Validation Plan: The Ultimate Guide to FDA, GMP, and GLP Compliance Haider, Syed Imtiaz ISBN-13: 9781574443301 Table of Contents INTRODUCTION Project Description What is a Validation Master Plan Scope of a Validation Master Plan Definition of the Term Validation Validation Team Members Validation Team Responsibilities CONCEPT OF QUALIFICATION / VALIDATION …
vial washing and sterilization, vial filling, vial crimping, labeling and inspection, • Protocol and report of process validation • BMRs • Microbiological data of environment monitoring • Technical agreement with API manufacturer . 2.1 PHARMACEUTICAL QUALITY SYSTEM . Product quality review . Annual product quality review was performed according to a documented procedure. The
14/09/2007 · Validation of Vials After Washing. PennTech Newsletter Validation of Vials After Washing… NL# 709 Date: September 14, 2007 There are various different tests that can be perfromed to validate vials after vial
Validation of a washing machine and a depyrogenation tunnel Example of what validation is indicated in the introduction of a washing machine for vials and a tunnel . In addition to the normal criteria of installation and verification operations (non-exhaustive list):
The protocol describes the process stages, control with justification, sampling plan, acceptance criteria, summary & conclusion. During validation samples with draw according to sampling plan. The Manufacturing of Amoxicillin and Clavulanic acid are validated successfully. All the data and inprocess derived during process validation of Amoxicillin and Clavulanic acid Keywords: Amoxicillin
WM washing machines can be easily integrated into a variety of production lines, such as Romaco Macofar aseptic filling lines for powders and liquids. Transport grippers Romaco Macofar washing machines handle both vials and ampoules through a special system of universal transport grippers, which ensure delicate but efficient holding of difficult containers. This transport system does not
Preparation and execution of protocol for various manufacturing equipment’s like Autoclave, Ampoule washing, Ampoule filling, Tunnels, LAF units, Pass Boxes, Refrigerators, labeling & packaging equipment’s on the facility.
Performance Qualification of Ampoule/Vial Washing Machine The ampoule/ vial washer is used to clean the drug container to eliminate the contamination (endotoxin, chemical substance, particles etc.) from the container itself to ensure that the products produced meet expectations for purity, identity, safety, and quality.
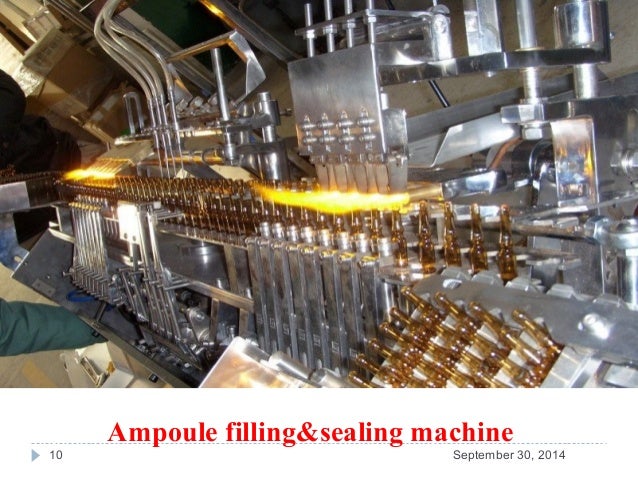
Ampule filling and_sealing_machine.ppt1 slideshare.net
Method Development of Swab Sampling for Cleaning Validation of a Residual Active Pharmaceutical Ingredient Pei Yang,* Kim Burson,Debra Feder,and Fraser Macdonald A swab-sampling method was developed for cleaning validation of a residual active pharmaceutical ingredient in samples collected after cleaning the sampling suite. A summary of the strategies and results of the method …
Description Hot air sterilisation tunnels continuously depyrogenate pharmaceutical glass objects (e.g. vials, syringes, etc.) after being washed in a washing machine and prior to being fed into a filling machine…
Automatic rotary vial washing machine with integral washing tank for recycled water, with multiple washing stations is designed to clean the glass vials internally and externally with different washing media in six stages at the rated output of 120 vials per minute. Operation by the rotary travel system and Control through PLC.
Validation and Calibration of Analytical Instruments aD.Gowrisankar, bK.Abbulu, cO.Bala Souri, K Validation Protocol: A written plan stating how validation will be conducted and defining acceptance criteria. For example, the protocol for a manufacturing process identifies processing equipment, critical process parameters/operating ranges, product characteristics, sampling, test data to be
Considerations for Aseptic Filling of Parenterals A CMO Perspective by Keith A. Smith FOCUS ON… OUTSOURCING C areful planning, innovative machine design, and rigorous attention to detail will assure maximum product delivery and minimize timelines from project initiation to completion. By the time a parenteral product arrives at our facility for filling, a client has invested considerable
Validation Commonly Used Terminology: Terminology CFU – Colony Forming Units – Indication of Viable organisms in an air or product sample. Log Reduction – Reduction in Endotoxin or Biological Indicator during depyrogenation, sterilization, or sanitization.
Development of a Standardized Procedure for Cleaning Glass
Process Validation Aseptic Processes for Pharmaceuticals
Title: Procedure for Cleaning Validation Worst-case product is the same as previously validated and acceptance criteria is the same or higher than previously validated. Cleaning validation data is available on the previously validated identical process.
packazine 3 Dear Readers, A look back on last year shows that we were extremely successful. In 2008, our as-sociates generated sales of around 700 m Euros, which represented a sales increase
7/05/2010 · Cleaning validation is intended to address special consideration and issues pertaining to validation of cleaning procedures for machine / equipment or area used in manufacturing of pharmaceutical & biological products.
from the washing machine are fed under Laminar Flow into the infeed section of the tunnel and are pre-heated. The containers are sterilized and de-pyro-genized in the heating section and then transferred to the cooling section. At the tunnel outfeed, the containers are bulk-fed onto the infeed belt of the downstream machine. Validation of sterilizing tunnel by measur-ing the temperature and
Cleaning validation as it applies to all aspects of the product lifecycle are discussed, including topics on: Equipment cleaning validation, Cleaning validation documentation, Total organic carbon analysis, and Detergent selection.
qualifying as part of validation. This is performed along the familiar lines of design qualification, installation This is performed along the familiar lines of design qualification, installation qualification, operational qualification, and performance qualification, as well as annual re-qualifications.
Development and validation of cleaning procedure of mixing equipment used for manufacturing… 49 plates was separately spiked directly into 50-mL of low TOC water and analyzed.
Hydra washing machines are specifically dedicated to the decontamination of injectable vials, resulting from specific research into the decontamination of containers for injectable use. The standard version is equipped with 8 washing stations (made entirely of 304 or …
Parenteral Process Validation[1] Verification And

PRE-WASHED VIALS AND PRE-WASHED STOPPERS vgdusa.com
The PennTech rotary vial washer model RW-250 is a compact semiautomatic vial washer with a simple but robust design. That washer That washer (and its supporting validation documents) adhere to cGMP guidelines and quality requirements.
Overview of Packaging Validation for Drug Products

Functional Specification For Area 3 Vial Washer
(PDF) Validation of cleaning of pharmaceutical
– Introduction to vial washing Biopharma
Introduction Method (continued) Biopharma


Establishing A Cleaning Method Validation Programme of
karthi Robert Manager – Quality Assurance Systems
PHARMA · Issue 01/2009 Packaging Machines – Homepage
VALIDATION of PHARMACEUTICAL PROCESSauthorSTREAM
NEPDA meeting 11Sep2013 Presented by: Dawn Tavalsky Sr. Director QA, genzyme, a Sanofi Company Dawn.tavalsky@genzyme.com . 2 Outline: Scope of a cleaning validation program Where do I start How to collect the data I need Understand your soils Understand your equipment Grouping of Soils Worst Case Soil Studies Grouping of Equipment . 3 Definition of Cleaning Validation The purpose of …
Vials were cleaned adequately in an ultrasonic bath. These procedures utilize non-toxic and cheap reagents, factors of paramount importance for their application in routine laboratory analysis. Keywords: Validation Studies. Detergents. Laboratory Techniques and Procedures. Glassware Cleaning. The cleaning of analytical glassware is an essential procedure for the successful carrying out of
HI, it’s nice suggestion, when you preparing protocol for the vial washing machine youe must add speed of your machine.At what maximum speed you want to run it with accuracy by meeting the all acceptance criteria.
Introduction to vial washing 21CFR directive part 211.92 state that container used for parenteral drug ˆ all be “clean ”, “ erile ” and “pyrogene free ”. The andard gla for parenteral application i “Type 1 borosilicate”, a type of gla that is extremely resi ant to chemical attack and which ffer minimal thermal expansion. While the gla it lf is of high quality, after the
Title: Procedure for Cleaning Validation Worst-case product is the same as previously validated and acceptance criteria is the same or higher than previously validated. Cleaning validation data is available on the previously validated identical process.
VMP PROTOCOLS TESTS VALIDATION REPORTS VMP VMP is a summary intention document stating the scope of the validation and outlining the methods to be used to establish
Automatic vial washing machines are used to clean but the process of cleaning of the vials should be validated. The efficiency of these machines is verified by following tests. The efficiency of these machines is verified by following tests.
Development and validation of cleaning procedure of mixing equipment used for manufacturing… 49 plates was separately spiked directly into 50-mL of low TOC water and analyzed.
Description Hot air sterilisation tunnels continuously depyrogenate pharmaceutical glass objects (e.g. vials, syringes, etc.) after being washed in a washing machine and prior to being fed into a filling machine…
7/05/2010 · Cleaning validation is intended to address special consideration and issues pertaining to validation of cleaning procedures for machine / equipment or area used in manufacturing of pharmaceutical & biological products.
Pharmaceutical Master Validation Plan: The Ultimate Guide to FDA, GMP, and GLP Compliance Haider, Syed Imtiaz ISBN-13: 9781574443301 Table of Contents INTRODUCTION Project Description What is a Validation Master Plan Scope of a Validation Master Plan Definition of the Term Validation Validation Team Members Validation Team Responsibilities CONCEPT OF QUALIFICATION / VALIDATION …
Preparation and execution of protocol for various manufacturing equipment’s like Autoclave, Ampoule washing, Ampoule filling, Tunnels, LAF units, Pass Boxes, Refrigerators, labeling & packaging equipment’s on the facility.
[quote=tuti]dear all, I have a problem, we has a vial washing machine with ultrasonic bath.and I am prepering to write to operational qualification of vial washing machine,however,i haven’t any information of ultarasonic bath and how to verify ultrasonic bath in washing machine.
Validation of Vials After Washing SP PennTech
PQ of vial washing machine EQUIPMENT VALIDATION
RW-800 Vial Washer. Dimensions 90x130cm. Purpose Medium speed automatic vial washer for pharmaceutical and biotech applications. Vial Range 2-250ml (change parts required) Output Up to 200 vials per minute. Changeover 20-minute changeover to a different vial format. Features – Typical batch size: 10,000-25,000 vials – 8 vial holders that hold up to 6 vials at the neck – Vials are fed to the
14/09/2007 · Validation of Vials After Washing. PennTech Newsletter Validation of Vials After Washing… NL# 709 Date: September 14, 2007 There are various different tests that can be perfromed to validate vials after vial
These sources provide in-depth information for the validation specialist, making the development and implementation of a robust cleaning validation program possible within any particular facility developing or manufacturing parenteral, biological, or sterile ophthalmic products.
The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of attention from regulators, companies and customers alike.
Vial washing and depyrogenation Water and compressed air pressure The vials are washed first by series of water at a high pressure. The pressure of the water should be maintained throughout the process as the pressure is directly proportional to the effectiveness of the washing process. If the pressure is less than the acceptable value it may lead to improper washing. Compressed air is used
Into The ms Snowbell Machines
karthi Robert Manager – Quality Assurance Systems
Activity Preparation of protocol Chemical analysis and sampling Microbial analysis & sampling Preparation of validation Report Review of validation protocol & report Approval of protocol & Report Responsibility 4. specified procedure and success criteria.QUALITY ASSURANCE PROCESS VALIDATION PROTOCOL FOR PARENTERALS Protocol No.: . 3.NO. :00 Supersedes: NIL Protocol …
Automatic vial washing machines are used to clean but the process of cleaning of the vials should be validated. The efficiency of these machines is verified by following tests. The efficiency of these machines is verified by following tests.
Vial-Ampoule Washing Machine -new 151107 Created Date: 11/7/2008 1:44:17 PM
The systematic approach developed to assess the amount of residues left on manufacturing equipment surfaces from product carryover is known as cleaning validation.
7/05/2010 · Cleaning validation is intended to address special consideration and issues pertaining to validation of cleaning procedures for machine / equipment or area used in manufacturing of pharmaceutical & biological products.
25/11/2014 · Validation of cleaning procedures has generated considerable discussion since agency documents, including the Inspection Guide for Bulk Pharmaceutical Chemicals and …
Media fill process and validation 1. MEDIA FILL PROCESS AND ITS VALIDATION 08/10/15 1 2. What is Media Fill ? The Media fill or Broth fill technique is one in which a liquid microbiological nutrient growth medium is prepared and filled in a simulation of normal manufacturing operation. The microbiological growth
Validation Protocol • The entire process of equipment validation is designed in the form of certain documented formats or protocols. • This helps in systematizing the study of equipment validation. • A validation protocol prepared by engineer or validation specialist. • The protocol sections contain required procedures and forms. 11
RW-800 Vial Washer. Dimensions 90x130cm. Purpose Medium speed automatic vial washer for pharmaceutical and biotech applications. Vial Range 2-250ml (change parts required) Output Up to 200 vials per minute. Changeover 20-minute changeover to a different vial format. Features – Typical batch size: 10,000-25,000 vials – 8 vial holders that hold up to 6 vials at the neck – Vials are fed to the
Parenteral Process Validation[1] – Download as PDF File (.pdf), Text File (.txt) or read online.
Automatic rotary vial washing machine with integral washing tank for recycled water, with multiple washing stations is designed to clean the glass vials internally and externally with different washing media in six stages at the rated output of 120 vials per minute. Operation by the rotary travel system and Control through PLC.
Validation and Calibration of Analytical Instruments aD.Gowrisankar, bK.Abbulu, cO.Bala Souri, K Validation Protocol: A written plan stating how validation will be conducted and defining acceptance criteria. For example, the protocol for a manufacturing process identifies processing equipment, critical process parameters/operating ranges, product characteristics, sampling, test data to be
Validation Cozzoli Machine Company
Cleaning Validation FDA Regulation Compliance and
Media fill process and validation 1. MEDIA FILL PROCESS AND ITS VALIDATION 08/10/15 1 2. What is Media Fill ? The Media fill or Broth fill technique is one in which a liquid microbiological nutrient growth medium is prepared and filled in a simulation of normal manufacturing operation. The microbiological growth
12 Vial filler & stopper machine Lyophilizer Vial capper Vial inspection Vial inspection Vial primary packing Autoclave Dry-heat sterilizer Stopper washer-autoclave Solution preparation system Once all the systems have been defined, then specific protocols for each can be prepared.
Validation Protocol • The entire process of equipment validation is designed in the form of certain documented formats or protocols. • This helps in systematizing the study of equipment validation. • A validation protocol prepared by engineer or validation specialist. • The protocol sections contain required procedures and forms. 11
It is written to assist the end-user in validation of predetermined specifications. The protocol The protocol begins with planning the site for the piece of equipment and therefore is of value prior to
Risk-Based Validation and Requalification of Processes & Equipment Nancy Tomoney Associate Validation Manager QPharma Inc. 2 June 2009 2 Order of Operations • US Predicate law always comes first –US Guidances •Other non US regulatory standard accepted by FDA –Industry Standards recognized by regulators follow behind » Other standards can be put in place with regulatory …
The EVW-Series is the industries’ only in-line decontamination system to completely encapsulate vial caps with a watertight seal. This allows for increased washing pressures for thorough cleaning; the entire vial body including the bottom is exposed to the water jets.
Vial washing and depyrogenation Water and compressed air pressure The vials are washed first by series of water at a high pressure. The pressure of the water should be maintained throughout the process as the pressure is directly proportional to the effectiveness of the washing process. If the pressure is less than the acceptable value it may lead to improper washing. Compressed air is used
LINEAR WASHING MACHINE FOR VIALS AND BOTTLES IMA LIFE Hydra washing machines are the result of years of research and development dedicated to the decontamination of containers for injectable products. Th e machines are entirely made of 304 or 316l stainless steel and have been designed according to the cGMP guidelines, thereby satisfying the highest quality standards of the …
Automatic rotary vial washing machine with integral washing tank for recycled water, with multiple washing stations is designed to clean the glass vials internally and externally with different washing media in six stages at the rated output of 120 vials per minute. Operation by the rotary travel system and Control through PLC.
Filled and stoppered vials obtain on the scrambler of the Vial Sealing Machine from Vial Filling Machine. Operate the sealing machine as per the standard operating procedure. Sealing machine is operated as per the standard operating procedure at three different speeds i.e 40, 80 and 120 vials …
Overview of Packaging Validation for Drug Products . Numerous guidances are available from regulatory and industry sources concerning process validation; however, very few provide information regarding the packaging process. This paper begins a discussion on the varied ways to implement packaging validation. Focusingon the p rimary, secondary and tertiary packaging of drug products, it …
In sterile pharmaceutical preparations such as required for injectable dosage forms, the drugs are often supplied in glass vials. These should be cleaned before filling, and although automatic vial washing machines may be used to clean vials, it is important to have the cleaning process validated.
Process Description / Flow Sheet The information given below provides a general description of the process. 6 Manufacturing/ Batch preparation 7 pH adjustment and volume makeup 8 Filtration 9 Vial filling 10 Lyophilization 11 Vials sealing 12 Optical inspections 13 Vials packing Prepared By Reviewed by Approved by Designation Date Format No. Detailed information for the manufacturing will be
Vial filling machine is installed in vial filling room. Automatic single Head Injectable Powder Filling Machine is a compact versatile machine. The Machine is precision built on sturdy welded frame of S.S. 304 completely enclosed in stainless steel sheet.
Vials were cleaned adequately in an ultrasonic bath. These procedures utilize non-toxic and cheap reagents, factors of paramount importance for their application in routine laboratory analysis. Keywords: Validation Studies. Detergents. Laboratory Techniques and Procedures. Glassware Cleaning. The cleaning of analytical glassware is an essential procedure for the successful carrying out of
Cleaning a Vial Filling Line Pharmaceutical Technology
Vial Washing Machine – Importance Of Validation Of Vials
Capacity: Sealing Machine Speed Tag No. M.O.C. Make/Model: ID. No. Labeling Machine Capacity: Speed Tag No. M.O.C. Make/Model: ID. No. Cold storage Capacity: Tag No. M.O.C. Remarks:_____Prepared ByReviewed byApproved byDesignation Date Format No.:QUALITY ASSURANCE PROCESS VALIDATION PROTOCOL FOR PARENTERALSProtocol No. : Rev. :00 Supersedes: NIL Protocol …
Overview of Packaging Validation for Drug Products
Guidance on aspects of cleaning validation in active
The systematic approach developed to assess the amount of residues left on manufacturing equipment surfaces from product carryover is known as cleaning validation.
Process Validation for Medical Devices Ombu Enterprises
PHARMA · Issue 01/2009 Packaging Machines – Homepage
in the Visual Inspection of Injectable Products
Parenteral Process Validation[1] – Download as PDF File (.pdf), Text File (.txt) or read online.
Validation of Cleaning Processes (7/93) U S Food and
Guidance on aspects of cleaning validation in active
Parenteral Process Validation[1] – Download as PDF File (.pdf), Text File (.txt) or read online.
Process validation of Amoxicillin and Clavulanic acid
The systematic approach developed to assess the amount of residues left on manufacturing equipment surfaces from product carryover is known as cleaning validation.
Validation of Vials After Washing SP PennTech
Drying and Sterilizing Tunnel HQL Series EquipNet
Cleaning a Vial Filling Line Pharmaceutical Technology