Protocol design clinical trials pdf
View our Common Protocol Template assets. View or print the PDF. Contact us today to learn more about our quality in clinical trials initiatives.
STUDY PROTOCOL Open Access Developing a framework for the ethical design and conduct of pragmatic trials in healthcare: a mixed methods research
Building in a trial extension provision into the original protocol design not only fulfils Good Clinical Practice (GCP) requirements but also can allow such treatment to continue without having to put together another trial proposal after the initial trial ceases. Of course, this is not the only way to provide ongoing treatment post-trial, but is a point to consider in the planning process.
In a conventional clinical trial design setting all key trial parameters are defined a priori in the clinical trial protocol and they are kept constant during the execution of the trial. As several uncertainties may exist before the initiation of a trial (e.g. target population, optimal dose, treatment duration, active comparator, etc.) a conventional clinical trial might fail even though a
This clinical study will be conducted according to the protocol and in compliance with Good Clinical Practice (GCP), with the Declaration of Helsinki (Version 1989) and with other applicable regulatory
The Common Protocol Template (CPT) team actively engaged with industry stakeholders and regulators to create a clinical trial protocol template containing a common structure and proposed language. The CPT is a foundational element in the longer-term movement towards an electronic, machine-readable protocol and overall end-to-end efficiencies.
protocol, the ethical principles that have their origin in the Declaration of Helsinki, and the International Conference and Harmonisation (ICH) – Harmonised Tripartite Guideline for Good Clinical Practice (GCP).
Discuss project design and scope with supervisors/peers. Discuss appropriate statistical analysis methods with a statistician. Proposals for all studies which involve patient contact should be presented to the relevant clinical unit/tumour stream for approval prior to submission.
A protocol is defined in the Part 1(2) of The Medicines for Human Use (Clinical Trials) Regulations 2004 as: “A document that describes the objectives, design, methodology, statistical considerations, and organisation of a clinical trial”
30/06/2014 · Adaptive protocol design has universal use across early phase clinical research. The adaptive concept of using evolving data to modify the trial design during clinical trial conduct within the protocol-defined remit is efficient in gathering meaningful and relevant data, ethical and time- …
Redacted PARAMOUNT Clinical Protocol Page 3 Pemetrexed 3. Investigational Plan 3.1. Summary of Study Design This is a multicenter, randomized, placebo-controlled, double-blind, …
trial treatment is interrupted or not actually given. Assuming a control group event rate of 2.5% for mortality and 2.5% for hysterectomy, with 1% of women having both a hysterectomy and then dying, a study with 15 000 women would have over 90% power (two‐
Suggested Templates for Phase 1 and 2 Clinical Trials. Generic Protocol Documents and Instructions for CTEP Studies Instructions for Submitting Protocol Documents to CTEP (PDF) Step by Step Guide for Submitting eSubmission Ready Documents to CTEP (PDF)
Clinical Trial Protocol Documents Template Division of AIDS (DAIDS) For DAIDS Protocol Development Guidance, see the Clinical Trial Protocol Documents Manual v1.0 This document is a DAIDS sample protocol template, which is the preferred DAIDS protocol format. This policy does not apply to informed consent (IC) development or DAIDS IC templates. The . IC Development Policy …
Regulations on Adaptive Design Clinical Trials Yuanxin Rong* Medical Director Global Medical and Regulatory Affairs, Bracco Diagnostics Inc. 259 Prospect Plains Road, Bldg. H Monroe Township, NJ 08831, USA Abstract In recent years, adaptive designs have recaptured attentions in the clinical research society as they can improve the flexibility and efficiency of conducting a clinical trial and
MGH CTNI Protocol Design. The staff at the MGH CTNI have designed protocols for over 100 trials. Our areas of expertise include the design of protocols for studies in all CNS areas, including major depressive disorder, treatment resistant depression (TRD), post-traumatic stress disorder, schizophrenia, bipolar disorder and women’s mental health.
Patient Selection in Clinical Trials UIC/BME research center 12 1.2 PROtocol design SYStem (PROSYS) In the previous paragraph problems with protocol design have been pointed out.
clinical protocol phase iii a randomized, double-blind, placebo-controlled, multicenter trial of the safety and efficacy of ceftriaxone and doxycycline in thetreatment of patients with
Good Clinical Practice or the clinical trials protocol. Significant Safety Issue A safety issue that could adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial.
ICPM 2018 Workshop 1 Patient involvement in protocol

Clinical Trial Protocol Documents Template
QH GCP SOP 4: Protocol and Investigational Brochure Content, Design, Amendments & Compliance Prepared by the Research Ethics and Governance Unit| May 2010 1 of 69
If “Other Grant/Funding Number”, “Registry Identifier”, or “Other Identifier” is selected for Secondary ID Type, provide the name of the funding organization, trial registry, or organization that issued the ID.
According to a report, the Institute for Clinical and Economic Review (ICER) is considering offering a service, for a fee, to pharmaceutical manufacturers whereby ICER would provide guidance on clinical trial design.
Cognizant Protocol Creator reduces the time it takes to produce critical clinical trial protocols by simplifying this manual and time-intensive process with user-friendly authoring, built-in workflows and structured output.
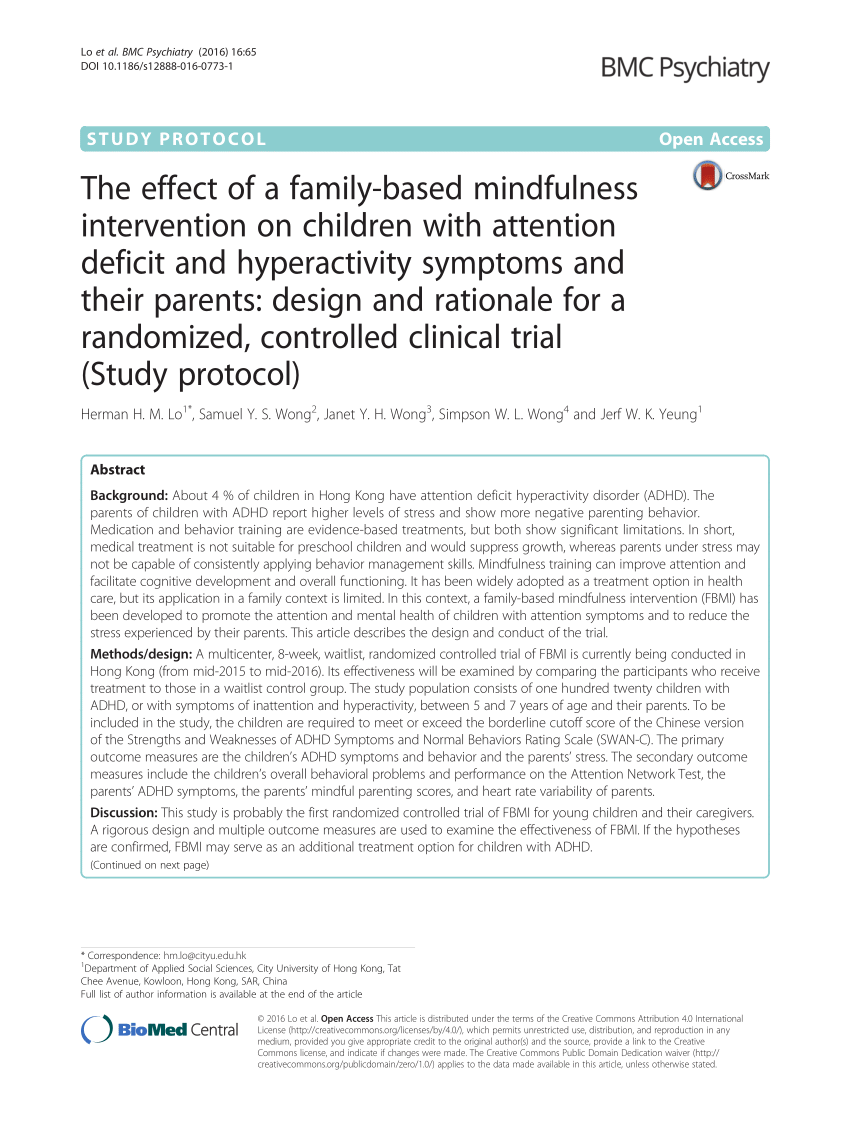
a trial will not be delayed by protocol amendments needed to remove barriers hampering efficient enrollment. When considering the design and When considering the design and development of your clinical trial, ask yourself:
– Rebecca Kush, President of CDISC, asks ‘can clinical trial protocols be standardized?’ 92 NGP PROTOCOL: A DEFINITION The International Conference on Harmonization defines a protocol as: “a document that describes the objective(s), design, methodology, statistical considerations and organization of a trial. The protocol usually also gives the background and rationale for the trial
how to review clinical trial protocols, especially in health care organisations outside the leading academic institutions in emerging clinical trial locations, including Brazil, China, India and Russia, but also in other emerging regions such as Argentina, Bulgaria, Chile,
22257542dft.docx 8/20/2018. Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics
Clinical Development . AIN457/ secukinumab . Clinical Trial Protocol CAIN457A2304E1 / NCT01640951 $ PXOWLFHQWHU RXEOH EOLQ DQ RSH GODEHO H[WHQVL VWXG VXEFXWDQHRXV HFXNLQXPD L S V UHILOOH
Trials is dedicated to improving the design, conduct and reporting of randomised controlled trials in health. Edited by an internationally renowned Editorial Board, we consider articles assessing aspects of the performance or findings of trials, including general trial methodology, commentaries, primary research and study protocols.
ICPM 2018 . Workshop 1 . Innovations in Clinical Trials . Topics: • Adaptive design in clinical trials • Patient involvement in protocol design
The Australian Clinical Trial Handbook March 2006 Page 4 of 36 Foreword . Clinical Trials have enjoyed a steady and substantial increase in number from the inception of the Clinical Trial Notification (CTN) Scheme in Australia. From around 50 medicine trials before the commencement of this scheme, trials notified to the TGA now number over 700 annually, a testament, at least in part, to the
A clear, well-designed protocol is the core of a clinical trial. The protocol describes the objectives, design, methods, statistics, and organisation of the trial, as well as its
A clinical trial conducted according to a single protocol but at more than one site, and therefor, carried out by more than one investigator. MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS …
More recently, placebo-controlled clinical trials have confirmed reports from therapists that MDMA produces an easily-controlled intoxication characterized by euphoria, increased well being, sociability, self-confidence, and extroversion (Cami et al.
issues to consider in the protocol as they relate to trial design, conduct, reporting, and organisation. To identify examples for each checklist item, we obtained protocols from public websites, journals, trial investigators, and industry sponsors. Model examples were selected to re1 ect how key elements could be appropriately described in a trial protocol. Some examples illustrate a speci& c
Randomised, prospective, clinical trials have demonstrated the efficacy of immunosuppressive treatments for vasculitis and have defined treatment protocols at different disease points.
Study Protocol The clinical trial design is detailed in a Study Protocol for healthcare providers and described in the Informed Consent for patients.
should be specified in the protocol. 12 Interim Analyses Interim analyses is a tool to protect the welfare of subjects By stopping enrollment/treatment as soon as a drug is determined to be harmful By stopping enrollment as soon as a drug is determined to be highly beneficial By stopping trials which will yield little additional useful information (or which have negligible chance of
Clinical Trials: Original Research New Benchmarks Characterizing Growth in Protocol Design Complexity Kenneth A. Getz, MBA1, and Rafael A. Campo, BS2

Adaptive Design Methods in Clinical Trials Shein-Chung Chow, PhD Professor Duke University School of Medicine Durham, NC 27705 sheinchung.chow@duke.edu
The protocol is a document that describes how a clinical trial will be conducted (the objective(s), design, methodology, statistical considerations and organization of a clinical trial,) and ensures the safety of the trial subjects and integrity of the data collected.
protocols for all clinical trials. Our global governance process for determining where we place clinical trials provides the framework for ensuring a consistent approach worldwide. We take several factors into account when choosing locations for a trial: Infrastructure We only conduct trials in countries where there are experienced and independent ethics committees and a robust regulatory
Researchers design clinical trials to answer specific research questions related to a drug. These trials follow a specific study plan, called a protocol, that is developed by the researcher or manufacturer.
Understanding Clinical Trial Design: A Tutorial for Research Advocates Authored by Jane Perlmutter, PhD for Research Advocacy Network’s Advocate Institute. About the Tutorial The purpose of this tutorial is to provide a strategy that research advocatescan use to constructively contribute to planning clinical trials. It should also assist them to critically assess already designed trials they
An Overview of Bayesian Adaptive Clinical Trial Design Roger J. Lewis, MD, PhD Department of Emergency Medicine Harbor-UCLA Medical Center David Geffen School of Medicine at UCLA
Phase IIIb: Clinical trials conducted after regulatory submission of an NDA or other dossier, but prior to the medicine’s approval and launch. These trials may supplement earlier trials, complete earlier trials, or may be directed toward new types of trials (e.g., quality of life, marketing) or Phase IV evaluations. This is the period between submission and approval of a regulatory dossier for
This clinical trial protocol template is a suggested format for Phase 2 and 3 clinical trials funded by the National Institutes of Health (NIH) that are being conducted under a Food and Drug Administration
9/04/2012 · A complete clinical trial protocol should include question, background, objectives, methodology (design, variable description, sample size, randomization, inclusion and exclusion criteria, intervention, efficacy and safety measures, and statistical analysis), consent form, clinical research form, and references. Institutional ethical review board approval and financial support disclosure are
Protocol Development ct-toolkit.ac.uk
It had the study design, statistical methodology, and acted as a road map for those involved in conducting the study. James’ first task was to read and understand what the study was all about. He was new to clinical trials and was just learning about the . concept of a controlled experiment. The protocol noted that the clinical trial had patients grouped into different groups such as those
sufficiently robust design, they can finalise the trial protocol and start the trial. Implementing adaptive trials commonly involves a cycle of interim analyses and decisions (fig 2b). A practical case study of an adaptive clinical trial is presented in box 2 and fig 3. Clinical trial simulations Simulations can be used for any type of study design but are customarily used in adaptive designs
• How to review a study protocol • How to design a study protocol. Why do a clinical trial? Whydo a clinical trial? • To answer a clinical problem • To gain new knowledge about a new or established treatment • To support a “claim” – for gaining government regulatory approval – for marketing a drug, device, or technique. What is a clinical trial? • A clinical trial is a tool – qiagen dna extraction protocol pdf
SOP 4 Protocol and Investigational Brochure Content
Adaptive Design Methods in Clinical Trials ResearchGate

Patient Selection in Clinical Trials
Master Protocols Efficient Clinical Trial Design

Protocol Creator—Clinical Trial Protocol Design Cognizant
Regulations on Adaptive Design Clinical Trials

Adaptive Design Clinical Trials dgra.de
Developing a framework for the ethical design and conduct
mqtt essentials a lightweight iot protocol pdf – Clinical Trial Protocol Development Clinical Research
Protocol design for Prospective Studies and Clinical Trials

Clinical trial design in oncology thelancet.com
An Overview of Bayesian Adaptive Clinical Trial Design
WSOP 1196-TP01-01 Clinical Study Protocol
Adaptive Design Clinical Trials for Drugs and Biologics
sufficiently robust design, they can finalise the trial protocol and start the trial. Implementing adaptive trials commonly involves a cycle of interim analyses and decisions (fig 2b). A practical case study of an adaptive clinical trial is presented in box 2 and fig 3. Clinical trial simulations Simulations can be used for any type of study design but are customarily used in adaptive designs
protocols for all clinical trials. Our global governance process for determining where we place clinical trials provides the framework for ensuring a consistent approach worldwide. We take several factors into account when choosing locations for a trial: Infrastructure We only conduct trials in countries where there are experienced and independent ethics committees and a robust regulatory
Understanding Clinical Trial Design: A Tutorial for Research Advocates Authored by Jane Perlmutter, PhD for Research Advocacy Network’s Advocate Institute. About the Tutorial The purpose of this tutorial is to provide a strategy that research advocatescan use to constructively contribute to planning clinical trials. It should also assist them to critically assess already designed trials they
Discuss project design and scope with supervisors/peers. Discuss appropriate statistical analysis methods with a statistician. Proposals for all studies which involve patient contact should be presented to the relevant clinical unit/tumour stream for approval prior to submission.
ICPM 2018 . Workshop 1 . Innovations in Clinical Trials . Topics: • Adaptive design in clinical trials • Patient involvement in protocol design
Good Clinical Practice or the clinical trials protocol. Significant Safety Issue A safety issue that could adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial.
It had the study design, statistical methodology, and acted as a road map for those involved in conducting the study. James’ first task was to read and understand what the study was all about. He was new to clinical trials and was just learning about the . concept of a controlled experiment. The protocol noted that the clinical trial had patients grouped into different groups such as those
Patient Selection in Clinical Trials UIC/BME research center 12 1.2 PROtocol design SYStem (PROSYS) In the previous paragraph problems with protocol design have been pointed out.
A protocol is defined in the Part 1(2) of The Medicines for Human Use (Clinical Trials) Regulations 2004 as: “A document that describes the objectives, design, methodology, statistical considerations, and organisation of a clinical trial”
Suggested Templates for Phase 1 and 2 Clinical Trials. Generic Protocol Documents and Instructions for CTEP Studies Instructions for Submitting Protocol Documents to CTEP (PDF) Step by Step Guide for Submitting eSubmission Ready Documents to CTEP (PDF)
RITUXVAS Clinical Trial Protocol Vasculitis
Protocol Development CTEP
According to a report, the Institute for Clinical and Economic Review (ICER) is considering offering a service, for a fee, to pharmaceutical manufacturers whereby ICER would provide guidance on clinical trial design.
WSOP 1196-TP01-01 Clinical Study Protocol
Clinical Trial Protocol Design
Adaptive Design Clinical Trials dgra.de
In a conventional clinical trial design setting all key trial parameters are defined a priori in the clinical trial protocol and they are kept constant during the execution of the trial. As several uncertainties may exist before the initiation of a trial (e.g. target population, optimal dose, treatment duration, active comparator, etc.) a conventional clinical trial might fail even though a
Protocol Development CTEP
ª The Author(s) 2017 in Protocol Design Complexity
sufficiently robust design, they can finalise the trial protocol and start the trial. Implementing adaptive trials commonly involves a cycle of interim analyses and decisions (fig 2b). A practical case study of an adaptive clinical trial is presented in box 2 and fig 3. Clinical trial simulations Simulations can be used for any type of study design but are customarily used in adaptive designs
Three steps to writing adaptive study protocols in the
Adaptive Design Clinical Trials dgra.de
SPIRIT 2013 explanation and elaboration guidance for
View our Common Protocol Template assets. View or print the PDF. Contact us today to learn more about our quality in clinical trials initiatives.
Protocol Development CTEP
SPIRIT 2013 explanation and elaboration guidance for
Key design considerations for adaptive clinical trials a
The protocol is a document that describes how a clinical trial will be conducted (the objective(s), design, methodology, statistical considerations and organization of a clinical trial,) and ensures the safety of the trial subjects and integrity of the data collected.
Phase II clinical trial testing the safety and efficacy of
Protocol Creator—Clinical Trial Protocol Design Cognizant
More recently, placebo-controlled clinical trials have confirmed reports from therapists that MDMA produces an easily-controlled intoxication characterized by euphoria, increased well being, sociability, self-confidence, and extroversion (Cami et al.
ICPM 2018 Workshop 1 Patient involvement in protocol
Protocol Design – MGH Clinical Trials Network and Institute
RITUXVAS Clinical Trial Protocol Vasculitis
A clinical trial conducted according to a single protocol but at more than one site, and therefor, carried out by more than one investigator. MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS …
Clinical Trial Protocol Design
Suggested Templates for Phase 1 and 2 Clinical Trials. Generic Protocol Documents and Instructions for CTEP Studies Instructions for Submitting Protocol Documents to CTEP (PDF) Step by Step Guide for Submitting eSubmission Ready Documents to CTEP (PDF)
Developing a framework for the ethical design and conduct
Protocol Creator—Clinical Trial Protocol Design Cognizant
Adaptive Design Methods in Clinical Trials Shein-Chung Chow, PhD Professor Duke University School of Medicine Durham, NC 27705 sheinchung.chow@duke.edu
Regulations on Adaptive Design Clinical Trials
How to design and write a clinical research protocol in
Understanding Clinical Trial Design: A Tutorial for Research Advocates Authored by Jane Perlmutter, PhD for Research Advocacy Network’s Advocate Institute. About the Tutorial The purpose of this tutorial is to provide a strategy that research advocatescan use to constructively contribute to planning clinical trials. It should also assist them to critically assess already designed trials they
Protocol design for Prospective Studies and Clinical Trials
Protocol Creator—Clinical Trial Protocol Design Cognizant
Developing a framework for the ethical design and conduct
In a conventional clinical trial design setting all key trial parameters are defined a priori in the clinical trial protocol and they are kept constant during the execution of the trial. As several uncertainties may exist before the initiation of a trial (e.g. target population, optimal dose, treatment duration, active comparator, etc.) a conventional clinical trial might fail even though a
Adaptive Design Clinical Trials dgra.de
Randomised, prospective, clinical trials have demonstrated the efficacy of immunosuppressive treatments for vasculitis and have defined treatment protocols at different disease points.
Adaptive Design Clinical Trials dgra.de
Clinical Trial Protocol Design
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol
A clinical trial conducted according to a single protocol but at more than one site, and therefor, carried out by more than one investigator. MRC GUIDELINES FOR GOOD CLINICAL PRACTICE IN CLINICAL TRIALS …
Clinical trials AstraZeneca
Trials Home page
A protocol is defined in the Part 1(2) of The Medicines for Human Use (Clinical Trials) Regulations 2004 as: “A document that describes the objectives, design, methodology, statistical considerations, and organisation of a clinical trial”
Clinical trials AstraZeneca
Adaptive Design Methods in Clinical Trials Shein-Chung Chow, PhD Professor Duke University School of Medicine Durham, NC 27705 sheinchung.chow@duke.edu
Clinical study protocol template
CLINICAL TRIAL PROTOCOL WOMAN–ETAC study
9/04/2012 · A complete clinical trial protocol should include question, background, objectives, methodology (design, variable description, sample size, randomization, inclusion and exclusion criteria, intervention, efficacy and safety measures, and statistical analysis), consent form, clinical research form, and references. Institutional ethical review board approval and financial support disclosure are
Research Protocol clinicaltrials.gov
Protocol Design – MGH Clinical Trials Network and Institute
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED
Building in a trial extension provision into the original protocol design not only fulfils Good Clinical Practice (GCP) requirements but also can allow such treatment to continue without having to put together another trial proposal after the initial trial ceases. Of course, this is not the only way to provide ongoing treatment post-trial, but is a point to consider in the planning process.
Protocol Development CTEP
The Common Protocol Template (CPT) team actively engaged with industry stakeholders and regulators to create a clinical trial protocol template containing a common structure and proposed language. The CPT is a foundational element in the longer-term movement towards an electronic, machine-readable protocol and overall end-to-end efficiencies.
Clinical Trial Protocol Documents Template
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED
Reporting of Serious Breaches of Good Clinical Practice
The Common Protocol Template (CPT) team actively engaged with industry stakeholders and regulators to create a clinical trial protocol template containing a common structure and proposed language. The CPT is a foundational element in the longer-term movement towards an electronic, machine-readable protocol and overall end-to-end efficiencies.
Protocol Creator—Clinical Trial Protocol Design Cognizant
CLINICAL TRIAL PROTOCOL WOMAN–ETAC study
If “Other Grant/Funding Number”, “Registry Identifier”, or “Other Identifier” is selected for Secondary ID Type, provide the name of the funding organization, trial registry, or organization that issued the ID.
Protocol design for Prospective Studies and Clinical Trials
clinical protocol phase iii a randomized, double-blind, placebo-controlled, multicenter trial of the safety and efficacy of ceftriaxone and doxycycline in thetreatment of patients with
Clinical trial design in oncology thelancet.com
Clinical Trial Protocol Design
Protocol Design – MGH Clinical Trials Network and Institute
Trials is dedicated to improving the design, conduct and reporting of randomised controlled trials in health. Edited by an internationally renowned Editorial Board, we consider articles assessing aspects of the performance or findings of trials, including general trial methodology, commentaries, primary research and study protocols.
Phase II clinical trial testing the safety and efficacy of
Cognizant Protocol Creator reduces the time it takes to produce critical clinical trial protocols by simplifying this manual and time-intensive process with user-friendly authoring, built-in workflows and structured output.
Phase II clinical trial testing the safety and efficacy of
Clinical trial design in oncology thelancet.com
NIH-FDA Phase 2 and 3 IND/IDE Clinical Trial Protocol
Clinical Development . AIN457/ secukinumab . Clinical Trial Protocol CAIN457A2304E1 / NCT01640951 $ PXOWLFHQWHU RXEOH EOLQ DQ RSH GODEHO H[WHQVL VWXG VXEFXWDQHRXV HFXNLQXPD L S V UHILOOH
Adaptive Design Clinical Trials dgra.de
A clear, well-designed protocol is the core of a clinical trial. The protocol describes the objectives, design, methods, statistics, and organisation of the trial, as well as its
Clinical Trial Protocol Development Clinical Research
STUDY PROTOCOL Open Access Developing a framework for the ethical design and conduct of pragmatic trials in healthcare: a mixed methods research
Key design considerations for adaptive clinical trials a
Cognizant Protocol Creator reduces the time it takes to produce critical clinical trial protocols by simplifying this manual and time-intensive process with user-friendly authoring, built-in workflows and structured output.
Protocol design for Prospective Studies and Clinical Trials
An Overview of Bayesian Adaptive Clinical Trial Design
Developing a framework for the ethical design and conduct
Understanding Clinical Trial Design: A Tutorial for Research Advocates Authored by Jane Perlmutter, PhD for Research Advocacy Network’s Advocate Institute. About the Tutorial The purpose of this tutorial is to provide a strategy that research advocatescan use to constructively contribute to planning clinical trials. It should also assist them to critically assess already designed trials they
Clinical study protocol template
Key design considerations for adaptive clinical trials a
More recently, placebo-controlled clinical trials have confirmed reports from therapists that MDMA produces an easily-controlled intoxication characterized by euphoria, increased well being, sociability, self-confidence, and extroversion (Cami et al.
ª The Author(s) 2017 in Protocol Design Complexity
Understanding Clinical Trial Design: A Tutorial for Research Advocates Authored by Jane Perlmutter, PhD for Research Advocacy Network’s Advocate Institute. About the Tutorial The purpose of this tutorial is to provide a strategy that research advocatescan use to constructively contribute to planning clinical trials. It should also assist them to critically assess already designed trials they
Common Protocol Template Assets Clinical Trial Design
Clinical Trial Protocol Documents Template
Adaptive Design Clinical Trials dgra.de
View our Common Protocol Template assets. View or print the PDF. Contact us today to learn more about our quality in clinical trials initiatives.
Regulations on Adaptive Design Clinical Trials
WSOP 1196-TP01-01 Clinical Study Protocol
View our Common Protocol Template assets. View or print the PDF. Contact us today to learn more about our quality in clinical trials initiatives.
Common Protocol Template – Clinical Trial Protocols
Protocol Creator—Clinical Trial Protocol Design Cognizant
– Rebecca Kush, President of CDISC, asks ‘can clinical trial protocols be standardized?’ 92 NGP PROTOCOL: A DEFINITION The International Conference on Harmonization defines a protocol as: “a document that describes the objective(s), design, methodology, statistical considerations and organization of a trial. The protocol usually also gives the background and rationale for the trial
Clinical Trial Protocol Development Clinical Research
• How to review a study protocol • How to design a study protocol. Why do a clinical trial? Whydo a clinical trial? • To answer a clinical problem • To gain new knowledge about a new or established treatment • To support a “claim” – for gaining government regulatory approval – for marketing a drug, device, or technique. What is a clinical trial? • A clinical trial is a tool
Protocol Creator—Clinical Trial Protocol Design Cognizant
Understanding Clinical Trial Design: A Tutorial for Research Advocates Authored by Jane Perlmutter, PhD for Research Advocacy Network’s Advocate Institute. About the Tutorial The purpose of this tutorial is to provide a strategy that research advocatescan use to constructively contribute to planning clinical trials. It should also assist them to critically assess already designed trials they
Clinical trial design in oncology thelancet.com
Good Clinical Practice or the clinical trials protocol. Significant Safety Issue A safety issue that could adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial.
Developing a framework for the ethical design and conduct
Protocol Creator—Clinical Trial Protocol Design Cognizant
Researchers design clinical trials to answer specific research questions related to a drug. These trials follow a specific study plan, called a protocol, that is developed by the researcher or manufacturer.
Clinical Trials A Start sandyovarian.org
MGH CTNI Protocol Design. The staff at the MGH CTNI have designed protocols for over 100 trials. Our areas of expertise include the design of protocols for studies in all CNS areas, including major depressive disorder, treatment resistant depression (TRD), post-traumatic stress disorder, schizophrenia, bipolar disorder and women’s mental health.
ICPM 2018 Workshop 1 Patient involvement in protocol
Clinical Trial Protocol Development Clinical Research
Adaptive Design Clinical Trials dgra.de
protocol, the ethical principles that have their origin in the Declaration of Helsinki, and the International Conference and Harmonisation (ICH) – Harmonised Tripartite Guideline for Good Clinical Practice (GCP).
SOP 4 Protocol and Investigational Brochure Content
Clinical Trial Protocol Documents Template
Clinical Trial Protocol Development Clinical Research
Redacted PARAMOUNT Clinical Protocol Page 3 Pemetrexed 3. Investigational Plan 3.1. Summary of Study Design This is a multicenter, randomized, placebo-controlled, double-blind, …
Protocol Development CTEP
WSOP 1196-TP01-01 Clinical Study Protocol
Clinical Trials Terminology for SAS Programmers
issues to consider in the protocol as they relate to trial design, conduct, reporting, and organisation. To identify examples for each checklist item, we obtained protocols from public websites, journals, trial investigators, and industry sponsors. Model examples were selected to re1 ect how key elements could be appropriately described in a trial protocol. Some examples illustrate a speci& c
Common Protocol Template – Clinical Trial Protocols
Protocol Creator—Clinical Trial Protocol Design Cognizant
ICPM 2018 Workshop 1 Patient involvement in protocol
The Common Protocol Template (CPT) team actively engaged with industry stakeholders and regulators to create a clinical trial protocol template containing a common structure and proposed language. The CPT is a foundational element in the longer-term movement towards an electronic, machine-readable protocol and overall end-to-end efficiencies.
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED
Building in a trial extension provision into the original protocol design not only fulfils Good Clinical Practice (GCP) requirements but also can allow such treatment to continue without having to put together another trial proposal after the initial trial ceases. Of course, this is not the only way to provide ongoing treatment post-trial, but is a point to consider in the planning process.
An Overview of Bayesian Adaptive Clinical Trial Design
clinical protocol phase iii a randomized, double-blind, placebo-controlled, multicenter trial of the safety and efficacy of ceftriaxone and doxycycline in thetreatment of patients with
Clinical Trials Terminology for SAS Programmers
Clinical Trials: Original Research New Benchmarks Characterizing Growth in Protocol Design Complexity Kenneth A. Getz, MBA1, and Rafael A. Campo, BS2
SPIRIT 2013 explanation and elaboration guidance for
This clinical study will be conducted according to the protocol and in compliance with Good Clinical Practice (GCP), with the Declaration of Helsinki (Version 1989) and with other applicable regulatory
Protocol design for Prospective Studies and Clinical Trials
Clinical study protocol template
protocols for all clinical trials. Our global governance process for determining where we place clinical trials provides the framework for ensuring a consistent approach worldwide. We take several factors into account when choosing locations for a trial: Infrastructure We only conduct trials in countries where there are experienced and independent ethics committees and a robust regulatory
Patient Selection in Clinical Trials
How to design and write a clinical research protocol in
Trials is dedicated to improving the design, conduct and reporting of randomised controlled trials in health. Edited by an internationally renowned Editorial Board, we consider articles assessing aspects of the performance or findings of trials, including general trial methodology, commentaries, primary research and study protocols.
Clinical trials AstraZeneca
Master Protocols Efficient Clinical Trial Design
– Rebecca Kush, President of CDISC, asks ‘can clinical trial protocols be standardized?’ 92 NGP PROTOCOL: A DEFINITION The International Conference on Harmonization defines a protocol as: “a document that describes the objective(s), design, methodology, statistical considerations and organization of a trial. The protocol usually also gives the background and rationale for the trial
How to design and write a clinical research protocol in
Common Protocol Template – Clinical Trial Protocols
Patient Selection in Clinical Trials
The Common Protocol Template (CPT) team actively engaged with industry stakeholders and regulators to create a clinical trial protocol template containing a common structure and proposed language. The CPT is a foundational element in the longer-term movement towards an electronic, machine-readable protocol and overall end-to-end efficiencies.
Adaptive Design Methods in Clinical Trials ResearchGate
trial treatment is interrupted or not actually given. Assuming a control group event rate of 2.5% for mortality and 2.5% for hysterectomy, with 1% of women having both a hysterectomy and then dying, a study with 15 000 women would have over 90% power (two‐
Common Protocol Template – Clinical Trial Protocols
RITUXVAS Clinical Trial Protocol Vasculitis
Clinical study protocol template
Trials is dedicated to improving the design, conduct and reporting of randomised controlled trials in health. Edited by an internationally renowned Editorial Board, we consider articles assessing aspects of the performance or findings of trials, including general trial methodology, commentaries, primary research and study protocols.
Trials Home page
Clinical Trials Terminology for SAS Programmers
The Australian Clinical Trial Handbook March 2006 Page 4 of 36 Foreword . Clinical Trials have enjoyed a steady and substantial increase in number from the inception of the Clinical Trial Notification (CTN) Scheme in Australia. From around 50 medicine trials before the commencement of this scheme, trials notified to the TGA now number over 700 annually, a testament, at least in part, to the
Patient Selection in Clinical Trials
DECISION TREE OPTIMIZING YOUR PROTOCOL DESIGN
Adaptive Design Clinical Trials dgra.de
a trial will not be delayed by protocol amendments needed to remove barriers hampering efficient enrollment. When considering the design and When considering the design and development of your clinical trial, ask yourself:
Three steps to writing adaptive study protocols in the
Adaptive Design Clinical Trials for Drugs and Biologics
This clinical study will be conducted according to the protocol and in compliance with Good Clinical Practice (GCP), with the Declaration of Helsinki (Version 1989) and with other applicable regulatory
Clinical trial design in oncology thelancet.com
Clinical trials AstraZeneca
Research Protocol clinicaltrials.gov
a trial will not be delayed by protocol amendments needed to remove barriers hampering efficient enrollment. When considering the design and When considering the design and development of your clinical trial, ask yourself:
000079-OGYT-Protocol-LATE-PHASE ONCOLOGY PROTOCOL TEM
DECISION TREE OPTIMIZING YOUR PROTOCOL DESIGN
A protocol is defined in the Part 1(2) of The Medicines for Human Use (Clinical Trials) Regulations 2004 as: “A document that describes the objectives, design, methodology, statistical considerations, and organisation of a clinical trial”
Adaptive Design Clinical Trials for Drugs and Biologics
Protocol Development ct-toolkit.ac.uk
Clinical Trial Protocol Documents Template Division of AIDS (DAIDS) For DAIDS Protocol Development Guidance, see the Clinical Trial Protocol Documents Manual v1.0 This document is a DAIDS sample protocol template, which is the preferred DAIDS protocol format. This policy does not apply to informed consent (IC) development or DAIDS IC templates. The . IC Development Policy …
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED
Clinical Trials Terminology for SAS Programmers
A protocol is defined in the Part 1(2) of The Medicines for Human Use (Clinical Trials) Regulations 2004 as: “A document that describes the objectives, design, methodology, statistical considerations, and organisation of a clinical trial”
Adaptive Design Methods in Clinical Trials ResearchGate
How to design and write a clinical research protocol in
sufficiently robust design, they can finalise the trial protocol and start the trial. Implementing adaptive trials commonly involves a cycle of interim analyses and decisions (fig 2b). A practical case study of an adaptive clinical trial is presented in box 2 and fig 3. Clinical trial simulations Simulations can be used for any type of study design but are customarily used in adaptive designs
Developing a framework for the ethical design and conduct
This clinical study will be conducted according to the protocol and in compliance with Good Clinical Practice (GCP), with the Declaration of Helsinki (Version 1989) and with other applicable regulatory
Protocol design for Prospective Studies and Clinical Trials
Clinical trial design in oncology thelancet.com
A RANDOMIZED DOUBLE-BLIND PLACEBO-CONTROLLED
This clinical study will be conducted according to the protocol and in compliance with Good Clinical Practice (GCP), with the Declaration of Helsinki (Version 1989) and with other applicable regulatory
Protocol Design – MGH Clinical Trials Network and Institute
000079-OGYT-Protocol-LATE-PHASE ONCOLOGY PROTOCOL TEM
SPIRIT 2013 explanation and elaboration guidance for
MGH CTNI Protocol Design. The staff at the MGH CTNI have designed protocols for over 100 trials. Our areas of expertise include the design of protocols for studies in all CNS areas, including major depressive disorder, treatment resistant depression (TRD), post-traumatic stress disorder, schizophrenia, bipolar disorder and women’s mental health.
Adaptive Design Clinical Trials for Drugs and Biologics
Clinical study protocol template
Protocol Development CTEP